A case report of trastuzumab dose in gastric cancer
Introduction
Trastuzumab (Herceptin®, F. Hoffman-La Roche) is approved for the treatment of metastatic HER2-positive gastric cancer. The Trastuzumab for Gastric Adenocarcinoma (ToGA) study was a randomized Phase III clinical trial evaluating chemotherapy with and without trastuzumab in patients with HER2-positive gastric cancer, as defined as FISH positive (HER2:CEP17 >2.0) or IHC 3+ (using Hofmann scoring criteria (1). Following a loading dose, patients randomized to the trastuzumab arm received trastuzumab 2 mg/kg/wk as was established as standard treatment in breast cancer (2). Patients randomly assigned to receive trastuzumab with chemotherapy had significantly improved survival and clinical outcome (hazard ratio 0.74, 95% CI, 0.60-0.91, P=0.0046) (3). Based on this positive study, trastuzumab with cisplatinum/5-FU-based chemotherapy is now standard of care for HER2-positive gastric cancer.
Here, we describe a patient with HER2-positive metastatic gastric adenocarcinoma who had progressed on the standard dose of trastuzumab, but then responded to a higher dose.
Case report
A 68-year-old man with metastatic gastric cancer to the mediastinum and cervical lymph nodes was initially diagnosed in September 2010 when he presented with supraclavicular adenopathy. Excisional biopsy (9/17/10) revealed poorly-differentiated metastatic adenocarcinoma. The tumor was positive for CK7, CK20, p53, and negative for CDX2, TTF-1, EGFR/kRAS, ALK, and PSA. He had widespread metastatic disease including metastases to lymph nodes in the neck, bilateral hila, mediastinum, and retroperitoneum, as well as multiple sites within the lumbar spine.
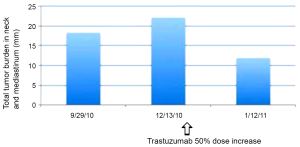
Upper endoscopy (10/19/2010) revealed distal esophageal thickening and biopsy of confirmed adenocarcinoma, positive for HER2 (FISH 3.0, IHC 2+) (DAKO). He began chemotherapy for metastatic HER2-positive gastroesophageal junction adenocarcinoma on 11/9/2010, receiving FOLFOX and trastuzumab (6 mg/kg load), followed by FOLFOX and trastuzumab 4 mg/kg every two weeks. However, after 3 cycles, on 12/13/10, the patient presented with increasing supraclavicular and neck adenopathy causing positional dyspnea. CT neck confirmed progressive lymphadenopathy involving every level of the neck. The trastuzumab dose was increased by 50% (6 mg every two weeks), and the FOLFOX chemotherapy remained unchanged. The patient quickly demonstrated clinical response with improvement in neck adenopathy and in resting dyspnea with a change in trastuzumab dose alone. CT CAP (1/21/11) demonstrated response with interval decrease in mediastinal, retrocrural, abdominal and upper retroperitoneal adenopathy. Figure 1 describes the cumulative tumor burden of his neck and upper chest adenopathy over time. The patient remained on therapy with FOLFOX and trastuzumab 6 mg/kg every 2 weeks with subsequent imaging demonstrating continued response to therapy (2/14/11, 4/14/11). The patient had progressive disease by June 2011, and died of advanced gastric cancer in August 2011.
Discussion
We present a case of HER2-positive metastatic gastric cancer that required a higher than standard dose of trastuzumab to achieve a response to therapy. Standard breast cancer dosing of trastuzumab on a 3-week schedule is 8 mg/kg load followed by 6 mg/kg q 3 weeks, or on a weekly schedule (4 mg/kg load, 2 mg/kg q week) (1). Our patient was treated with FOLFOX chemotherapy every two weeks, and thus received an appropriate proportional trastuzumab dose (6 mg/kg load, 4 mg/kg q 2 weeks). The patient progressed very quickly following initiation of therapy (after 3 treatments), and subsequently responded immediately following an increase in trastuzumab dose to 6 mg/kg q 2 weeks (i.e., 50% dose increase in maintenance). This response was noted without a change in the FOLFOX cytotoxic therapy, suggesting that the initial administered dose of trastuzumab was insufficient for treatment response; more specifically, the patient required a higher dose of trastuzumab to achieve a response to therapy.
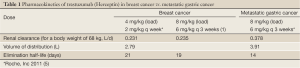
The observation that our patient responded to a higher dose of trastuzumab than routinely administered suggests that some patients with HER2-positive gastric cancer may be underdosed. It is suggested that gastric cancer patients may have a higher renal clearance of trastuzumab than patients with HER2-positive breast cancer. Bruno and colleagues (4) determined the steady state pharmacokinetics of trastuzumab in patients with metastatic breast cancer. On the weekly trastuzumab schedule, trastuzumab clearance is 0.231 L/day (for a median body weight of 68 kg) with a corresponding elimination half-life of approximately 3 weeks. On the every 3-week schedule in metastatic breast cancer, the trastuzumab pharmacokinetics is very similar (1). In contrast, the pharmacokinetic profile of trastuzumab reported from the ToGA study in patients with metastatic gastric cancer demonstrate a higher clearance is 0.378 L/day (~70% higher), with a corresponding elimination half-life of approximately only 2 weeks (Roche, Inc 2011) (Table 1) (5). This suggests that the current “standard” dosing of trastuzumab in metastatic gastric cancer may be grossly underdosed by nearly 50%, and that higher trastuzumab doses may be necessary in some patients for maximum efficacy.
Full Table
In breast cancer, it has been shown that patients with four or more metastatic sites of disease have faster clearance, independent of HER-2 extracellular domain levels (4). Trastuzumab elimination appears to depend on serum levels of circulating HER-2 extracellular domains, which can be cleaved from the surfaces of cancer cells by matrix metalloproteinase. While the relationship between circulating HER2neu extracellular domains (shed antigen) and tumor burden is unknown, it is reasonable to expect higher HER2 levels to be associated with higher tumor burden. This implies that patients with high HER-2 extracellular domain levels tend to have a shorter trastuzumab half-life and lower minimum concentrations (6). Together, these data suggest that many patients with gastric cancer with a high disease burden may be associated with a higher clearance of trastuzumab due to increased levels of shed Her-2 antigen. Consistent with this argument, our patient had a high disease burden with his primary tumor unresected, and with multiple metastases to bone and widespread adenopathy involving bilateral neck, mediastinum, and retroperitoneum.
Primary or acquired resistance to trastuzumab presents another possibility of compromised therapeutic efficacy. Resistance to trastuzumab will invariably develop in patients with advanced cancers treated with trastuzumab-containing regimens. Indeed, the rate of primary resistance to single-agent trastuzumab in HER2-overexpressing metastatic breast carcinomas is 66-88% (7-9). Proposed mechanisms of resistance in breast cancer include activation of multiple downstream signaling pathways (such as P13K/AKT pathway) (10), disruption of the interaction between the therapeutic agent and the target protein (11), and loss of the binding site on truncated HER2 receptors (12,13). There are currently no data regarding resistance mechanisms to trastuzumab in gastric cancer and no currently available in vitro tests available that effectively predict trastuzumab resistance in gastric cancer (14).
This case highlights that a higher dosing of trastuzumab may be necessary to compensate for increased renal clearance of the drug in metastatic gastric adenocarcinoma. Currently, trastuzumab’s elimination pathways are not clearly defined and the clinical relevance of trastuzumab’s kinetic variability is unknown. This is the subject of an ongoing international phase III study examining standard dosing versus high dosing trastuzumab + chemotherapy in metastatic HER2-positive gastric cancer (HELOISE Study) (NCT01450696 on www.clinicaltrials.gov). Although provocative, best practice suggests that we continue with standard dosing of trastuzumab until the results of the HELOISE study are available.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Hofmann M, Stoss O, Shi D, et al. Assessment of a HER2 scoring system for gastric cancer: results from a validation study. Histopathology 2008;52:797-805. [PubMed]
- Leyland-Jones B, Gelmon K, Ayoub JP, et al. Pharmacokinetics, safety, and efficacy of trastuzumab administered every three weeks in combination with paclitaxel. J Clin Oncol 2003;21:3965-71. [PubMed]
- Bang YJ, Van Cutsem E, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet 2010;376:687-97. [PubMed]
- Bruno R, Washington CB, Lu JF, et al. Population pharmacokinetics of trastuzumab in patients with HER2+ metastatic breast cancer. Cancer Chemother Pharmacol 2005;56:361-9. [PubMed]
- Roche, Inc. Herceptin package insert. Available online: http://www.medsafe.govt.nz/profs/datasheet/h/Herceptininf.pdf (accessed 10/8/2011)
- Meza-Junco J, Au HJ, Sawyer MB. Trastuzumab for gastric cancer. Expert Opin Biol Ther 2009;9:1543-51. [PubMed]
- Baselga J, Tripathy D, Mendelsohn J, et al. Phase II study of weekly intravenous recombinant humanized anti-p185HER2 monoclonal antibody in patients with HER2/neu-overexpressing metastatic breast cancer. J Clin Oncol 1996;14:737-44. [PubMed]
- Cobleigh MA, Vogel CL, Tripathy D, et al. Multinational study of the efficacy and safety of humanized anti-HER2 monoclonal antibody in women who have HER2-overexpressing metastatic breast cancer that has progressed after chemotherapy for metastatic disease. J Clin Oncol 1999;17:2639-48. [PubMed]
- Vogel CL, Cobleigh MA, Tripathy D, et al. Efficacy and safety of trastuzumab as a single agent in first-line treatment of HER2-overexpressing metastatic breast cancer. J Clin Oncol 2002;20:719-26. [PubMed]
- Lu CH, Wyszomierski SL, Tseng LM, et al. Preclinical testing of clinically applicable strategies for overcoming trastuzumab resistance caused by PTEN deficiency. Clin Cancer Res 2007;13:5883-8. [PubMed]
- Price-Schiavi SA, Jepson S, Li P, et al. Rat Muc4 (sialomucin complex) reduces binding of anti-ErbB2 antibodies to tumor cell surfaces, a potential mechanism for herceptin resistance. Int J Cancer 2002;99:783-91. [PubMed]
- Scaltriti M, Rojo F, Ocaña A, et al. Expression of p95HER2, a truncated form of the HER2 receptor, and response to anti-HER2 therapies in breast cancer. J Natl Cancer Inst 2007;99:628-38. [PubMed]
- Garnock-Jones KP, Keating GM, Scott LJ. Trastuzumab: A review of its use as adjuvant treatment in human epidermal growth factor receptor 2 (HER2)-positive early breast cancer. Drugs 2010;70:215-39. [PubMed]
- Ocaña A, Cruz JJ, Pandiella A. Trastuzumab and antiestrogen therapy: focus on mechanisms of action and resistance. Am J Clin Oncol 2006;29:90-5. [PubMed]


