Long-term survival in an advanced gastric cancer patient treated with cetuximab in association with FOLFIRI: a case report
Introduction
Treatment of advanced gastric cancer, traditionally with double or triple cytotoxic chemotherapy regimens, involves an advantage in overall survival of about 7-11 months compared to best supportive care (1). Though some data have emerged from a recent meta-analysis (2), there is currently no standard of treatment in the gastric cancer first-line setting. Again, at the time we were deciding how to treat our patient one was unable to use trastuzumab in metastatic gastric or gastroesophageal junction (GEJ) cancer HER2 positive, resulting later in a significant benefit in combination with cisplatin and 5-FU or capecitabine vs. chemotherapy alone (3).
Starting from gene expression tumor profiling, and given the presence of epidermal growth factor (EGF) in 25-30% of gastric cancer as well as the positive experience obtained in the metastatic colorectal cancer (mCRC) setting (4), we were prompted to investigate anti-EGFR therapy in gastric and GEJ cancer. Epidermal growth factor receptor (EGFR) is over expressed in 18-81% of gastric cancer, representing an unfavorable prognostic marker in multivariate data, typically associated with older age, more aggressive histology, higher stage disease and shorter survival. Tumors exhibiting EGF and EGFR simultaneously show a greater degree of local invasion and lymph node metastasis.
Case report
A 52-year old woman with recurrent epigastric pain and significant weight loss underwent esophagogastroduodenoscopy which revealed a large ulcerated lesion in the gastric antrum-body. Pre-operative radiological investigations did not show any metastatic disease. In November 2003, the patient underwent total gastrectomy with omentectomy and D2 lymphadenectomy, mechanical end-to-side anastomosis of the jejunal loop excluded by Roux. The antral region proved to have a macroscopic ulcerative vegetating lesion of about 6 cm infiltrating the wall and extending to the sierosa and adipose perigastric tissue. Histological examination gave evidence of intestinal adenocarcinoma, poorly differentiated and with focal areas of mucoid (pT3N3M0-Stage IIIA, G3; p53 100%, Ki67 52%, EGFR overexpressed).
From December 2003 to May 2004 adjuvant chemotherapy with a modified PELF regimen was performed to a total of six cycles.
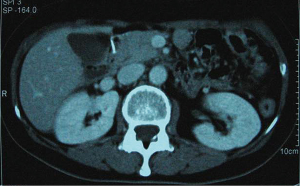
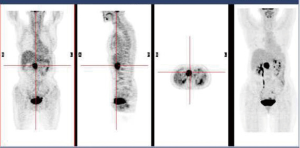
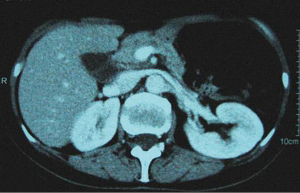
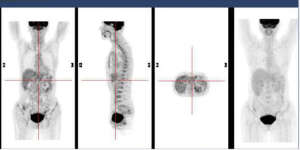
In December 2004 during a clinical follow-up, CT and 18F-FDG-PET-CT showed a retroperitoneal lymph node relapse in the form of a homogeneous solid mass sited at the pancreatic uncinate process, the maximum diameter being 5 cm (Figure 1), with SUVmax =18 at PET-CT (Figure 2). As a candidate for first-line chemotherapy treatment, she was enrolled in the phase II clinical trial FOLCETUX, receiving cetuximab at an initial dose of 400 mg/m2 i.v. followed by weekly doses of 250 mg/m2, irinotecan 180 mg/m2 i.v. on day 1, LFA 100 mg/m2 i.v. followed by 5-FU 400 mg/m2 i.v. bolus and 600 mg/m2 i.v. 22-h continuous infusion on days 1 and 2 every two weeks, to a total of 17 cycles. CT and PET-CT performed after six weeks treatment failed to show any residual disease, with complete radiological (Figure 3) response in accord to RECIST criteria and complete metabolic response (Figure 4). A total of 24 maintenance administrations with cetuximab alone (250 mg/m2 weekly) were performed, as foreseen by the protocol in responders. A grade 3 skin rash was observed during treatment.
In November 2005 elevated serum transaminases (AST =289 U/L; ALT =321 U/L) and subsequent diagnosis of HCV infection led to suspension of the cetuximab maintenance. The total body CT and PET-CT imaging continued to show no residual metabolic disease at the end of treatment.
In December 2007, since clinical and radiological response continued to be complete, treatment with interferon and ribavirin was started, and discontinued in January 2009.
In November 2012 a clinical, radiological (CT) and metabolic (PET-CT) patient examination proved negative for recurrent disease, signifying 95 months’ progression free survival.
Discussion
Cetuximab, the partially humanized murine anti-EGFR monoclonal antibody, has been the most examined anti-EGFR therapy in gastric cancer. It has low activity as a single agent (5), but the trend is different when it is added to single or double chemotherapy regimens. Eleven non-randomized first line phase II studies (6-16) have evaluated the activity and safety of cetuximab combined with different chemotherapy regimens, showing a response rate ranging from 38-69%, time to progression from 5.0 to 11 months and median overall survival between 8.6 and 16.6 months (Table 1).

Full table
As to what is the best chemotherapy regimen combination including cetuximab, there are no answers based on statistical significance, though the clinical results indicate substantial benefit when using irinotecan.
Tolerance of treatment and quality of life are of considerable importance in patients with advanced gastric cancer because most of them are symptomatic at baseline. Irinotecan monotherapy is active in gastric cancer patients with a phase II trial response rate of about 14-23%. This drug is more active when administered with 5-FU/folinic acid, and in two phase II trials achieves an overall response rate of 21-40% as well as median overall survival times of 6.4-11.3 months (17,18). In a large phase III study conducted by Dank et al., irinotecan plus 5-FU regimen showed a time-to-progression trend that was superior to cisplatin plus 5-FU: 5.0 versus 4.2 months, similar overall response rate (31.8% versus 25.8%) and median overall survival time (9.0 versus 8.7 months), but a better safety and toxicity profile.
In the FOLCETUX study the addition of cetuximab to the FOLFIRI regimen resulted in a median survival time of 16.6 months, longer time to progression and also an acceptable level of safety and a shorter time-to-response (six weeks) (6). These promising results prompted the German group to conduct a biomarker-oriented phase II study using the same combination but with a different administration schedule. Over a period of one year, a total of 49 patients enrolled achieved an overall response rate of about 46%; The disease control rate was 79%, median PFS and OS were 9.0 and 16.5 months, comparable with previously reported findings. The paper published by Moehler et al., as expected contained a pre-planned analysis of biomarkers involved in treatment outcomes using anti-EGFR targeted agents. The final data confirmed most of the analysis later carried out by us (19): the frequency of KRAS, BRAF and PIK3CA activating mutations found was very low. Unlike mCRC, where KRAS tumor mutation frequency is approximately 40%, and hence a negative prognostic and predictive factor of response to treatment with cetuximab, in gastric cancer KRAS mutation status seems to be an unsuitable predictive marker of cetuximab efficacy.
High hopes were placed in the EXPAND study presented at ESMO 2012, a large open-label, randomized, controlled phase III trial of cetuximab in combination with capecitabine and cisplatin in patients with advanced gastric cancer (20).
The results of the study failed to show benefit from the addition of cetuximab. The study protocol was terminated early due to the low progression-free survival observed. Between June 2008 and December 2010, 904 patients from 25 countries were enrolled and randomized, 455 patients received capecitabine, cisplatin and cetuximab while 449 received only cisplatin and capecitabine. Patient outcomes were similar between treatment groups, in that the primary and secondary endpoints were not met, progression-free survival was 4.4 compared to 5.6 months and overall survival was 9.4 compared to 10.7 months (respectively in the cetuximab-combination and control groups). The overall response rate was respectively 29% and 30%. Although toxicity grade 3/4 events and serious adverse reactions were reported more in the cetuximab-containing arm, the negative results of this process cannot only be explained by the increase in toxicity rates. Perhaps excessive enthusiasm deriving from the results obtained in small phase II trials inflated the importance of a randomized multicenter investigation into this, the best chemotherapy association previously tested.
The advantage of biological material stored in 97% of patients and currently under study is that EXPAND was a large study in a metastatic setting, performed in a homogeneous patient population, where the clinical database is of high quality, permitting translational research and establishing future subgroups of different types of gastric cancer based on gene expression profiling.
We must not forget that antibody drugs trigger intracellular cascades that can be augmented by chemotherapy association, for which reason perhaps the same holds for trastuzumab in combination with cisplatin and 5-FU or capecitabine does not apply to cetuximab, which is more effective for enhancing tumor shrinkage when combined with irinotecan, as has emerged in wild type KRAS mCRC.
When investigating the role of prognostic and predictive markers in an aggressive and disabling disease such as advanced gastric cancer, it is mandatory to define the patient setting clarifying who can obtain the most clinical benefit from the various biological and chemotherapy combination therapies.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Dank M, Zaluski J, Barone C, et al. Randomized phase III study comparing irinotecan combined with 5-fluorouracil and folinic acid to cisplatin combined with 5-fluorouracil in chemotherapy naive patients with advanced adenocarcinoma of the stomach or esophagogastric junction. Ann Oncol 2008;19:1450-7. [PubMed]
- Wagner AD, Unverzagt S, Grothe W, et al. Chemotherapy for advanced gastric cancer. Cochrane Database Syst Rev 2010;CD004064. [PubMed]
- Bang YJ, Van Cutsem E, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet 2010;376:687-97. [PubMed]
- Van Cutsem E, Köhne CH, Láng I, et al. Cetuximab plus irinotecan, fluorouracil, and leucovorin as first-line treatment for metastatic colorectal cancer: updated analysis of overall survival according to tumor KRAS and BRAF mutation status. J Clin Oncol 2011;29:2011-9. [PubMed]
- Chan JA, Blaszkowsky LS, Enzinger PC, et al. A multicenter phase II trial of single-agent cetuximab in advanced esophageal and gastric adenocarcinoma. Ann Oncol 2011;22:1367-73. [PubMed]
- Pinto C, Di Fabio F, Siena S, et al. Phase II study of cetuximab in combination with FOLFIRI in patients with untreated advanced gastric or gastroesophageal junction adenocarcinoma (FOLCETUX study). Ann Oncol 2007;18:510-7. [PubMed]
- Woell E, Greil R, Eisterer W, et al. Oxaliplatin,irinotecan, and cetuximab in advanced gastric cancer. First efficacy results of a multicenter phase II trial (AGMT Gastric-2). J Clin Oncol 2008;26:abstr 15587.
- Pinto C, Di Fabio F, Barone C, et al. Phase II study of cetuximab in combination with cisplatin and docetaxel in patients with untreated advanced gastric or gastro-oesophageal junction adenocarcinoma (DOCETUX study). Br J Cancer 2009;101:1261-8. [PubMed]
- Han SW, Oh DY, Im SA, et al. Phase II study and biomarker analysis of cetuximab combined with modified FOLFOX6 in advanced gastric cancer. Br J Cancer 2009;100:298-304. [PubMed]
- Kanzler S, Trarbach T, Seufferlein T, et al. Cetuximab with irinotecan/folinic acid/5-FU as first-line treatment in advanced gastric cancer: a nonrandomized multicenter AIO phase II study. J Clin Oncol 2009;27:abstr 4534.
- Yeh K, Hsu C, Hsu C, et al. Phase II study of cetuximab plus weekly cisplatin and 24-hour infusion of high-dose 5-fluorouracil and leucovorin for the first-line treatment of advanced gastric cancer. J Clin Oncol 2009;27:abstr 4567.
- Zhang X, Xu J, Shen L, et al. A phase II study of cetuximab with cisplatin and capecitabine as firstline treatment in advanced gastric cancer. J Clin Oncol 2008;26:abstr 15663.
- Lordick F, Luber B, Lorenzen S, et al. Cetuximab plus oxaliplatin/leucovorin/5-fluorouracil in first-line metastatic gastric cancer: a phase II study of the Arbeitsgemeinschaft Internistische Onkologie (AIO). Br J Cancer 2010;102:500-5. [PubMed]
- Enzinger PC, Burtness B, Hollis D, et al. CALGB 80403/ECOG 1206: a randomized phase II study of three standard chemotherapy regimens (ECF, IC, FOLFOX) plus cetuximab in metastatic esophageal and GE junction cancer. J Clin Oncol 2010;28:abstr 4006.
- Moehler M, Mueller A, Trarbach T, et al. Cetuximab with irinotecan, folinic acid and 5-fluorouracil as first-line treatment in advanced gastroesophageal cancer: a prospective multi-center biomarker-oriented phase II study. Ann Oncol 2011;22:1358-66. [PubMed]
- Kim C, Lee JL, Ryu MH, et al. A prospective phase II study of cetuximab in combination with XELOX (capecitabine and oxaliplatin) in patients with metastatic and/or recurrent advanced gastric cancer. Invest New Drugs 2011;29:366-73. [PubMed]
- Bouché O, Raoul JL, Bonnetain F, et al. Randomized multicenter phase II trial of a biweekly regimen of fluorouracil and leucovorin (LV5FU2), LV5FU2 plus cisplatin, or LV5FU2 plus irinotecan in patients with previously untreated metastatic gastric cancer: a Federation Francophone de Cancerologie Digestive Group Study--FFCD 9803. J Clin Oncol 2004;22:4319-28. [PubMed]
- Moehler M, Eimermacher A, Siebler J, et al. Randomised phase II evaluation of irinotecan plus high-dose 5-fluorouracil and leucovorin (ILF) vs 5-fluorouracil, leucovorin, and etoposide (ELF) in untreated metastatic gastric cancer. Br J Cancer 2005;92:2122-8. [PubMed]
- Pinto C, Mutri V, Di Fabio F, et al. Analysis of biomarker expression (Ki 67, EGFR, HER2, ERK, NFkB and mTOR) in patients with advanced gastric and gastroesophageal junction (GEJ) cancer treated with cetuximab plus cisplatin/docetaxel: DOCETUX study. J Clin Oncol 2010;28:abstr 4133.
- Lordick F, Kang YK, Chung HC, et al. Capecitabine and cisplatin with or without cetuximab for patients with previously untreated advanced gastric cancer (EXPAND): a randomised, open-label phase 3 trial. Lancet Oncol 2013;14:490-9. [PubMed]