Fourth versus eighth week surgery after neoadjuvant radiochemotherapy in T3-4/N0+ rectal cancer: Istanbul R-01 study
Introduction
Several decades ago, surgery alone was the standard treatment for locally advanced rectal cancer, which was associated with high rates of pelvic recurrence resulting in significant morbidity and mortality (1). This led to the idea of adding radiotherapy and/or chemotherapy to surgery in order to obtain better local control and possibly improved survival rates. Several trials compared the efficacy and safety of different treatment modalities in an attempt to define an optimal treatment strategy in terms of efficacy and safety. In late 80’s, addition preoperative radiotherapy has been shown to decrease local recurrences (2). Radiotherapy alone or in combination with chemotherapy has been tested in the randomized EORTC-22921 study, which established that neoadjuvant radiotherapy is not enough for local control and neoadjuvant radiochemotherapy should be the standard for clinically resectable rectal cancers owing to its better control rates (3). Other studies showed the superiority of preoperative radiotherapy over postoperative therapy (4), with no additional postoperative morbidity (5). Based on this growing body of evidence, neoadjuvant radiochemotherapy and transmesorectal excision (TME) has become the accepted therapeutic modality in clinical stage T3 and N0/+ patients.
Although most surgeons prefer waiting four to eight weeks after neoadjuvant chemoradiotherapy (6), the optimum duration has not been defined yet. Since recovery of mesorectum needs time, increasing the interval between two treatments has the potential to enhance outcomes. Delaying the interval up to 14 weeks does not seem to compromise safety (7). To date, several studies compared shorter versus longer delays after chemoradiotherapy with conflicting findings in terms of local control and survival (6,8-10).
Circumferential margin (CRM) positivity is still major reason for local recurrences and systemic failure in rectal cancers (11). In addition to local recurrence and survival rates, circumferential margin (CRM) positivity represents another important endpoint to evaluate the effectiveness of neoadjuvant treatment, since most local relapses originate from the surgical margin.
This Istanbul R0-1 prospective randomized study was designed to compare the efficacy of four-week (4 w) versus eight-week (8 w) delay before surgery after concomitant neoadjuvant chemoradiotherapy in terms of local recurrence, circumferential margin positivity, and overall survival in cT3-4/N0+, mid- and distally localized (intraperitoneal) rectal cancers.
Material and methods
Patient eligibility and enrollment
To be eligible for this single center prospective randomized trial, patients had to present with locally advanced (T3-4 or N0/N+) low- (Level I or below 59 mm from the anal verge) or mid-rectum (Level II or 60-119 mm from the anal verge) rectal adenocarcinoma. Exclusion criteria included secondary malignancy, inflammatory bowel disease, uncontrolled diabetes or infection, pregnancy, and an ECOG performance status greater than 2. The study protocol was approved by Surgical Review Board of Istanbul University, Istanbul Medical Faculty. Study procedures were in accordance with Declaration of Helsinki and all patients gave informed consent prior to study entry. Primary endpoint was local recurrence and secondary endpoints were overall survival and circumferential margin positivity.
Randomization and treatments
Patients were randomly assigned into two groups: Group A (4 w) and Group B (8 w). All patients received neoadjuvant chemoradiotherapy prior to surgery. Patients in Group A (4 w) underwent total mesorectal excision (TME) with curative intent four weeks after neoadjuvant therapy, whereas patients in Group B (8 w) received surgery after eight weeks.
For pretreatment staging, flexible colonoscopy, endorectal ultrasonography (EUS), computerized tomography (CT) or magnetic resonance imaging (MRI) of the pelvis were used. In addition, abdominal or thoracic CT was used to rule out distant metastasis.
The neoadjuvant radiotherapy regimen included 45 cGy radiation delivered to the posterior pelvis in 25 fractions (1.8 Gy per fraction) over 5 weeks. Neoadjuvant chemotherapy consisted of 225 mg/m2·day 5-fluorouracil infusion using catheter or implantofix over the same 5 weeks. All patients were examined every week by attending physicians during chemoradiotherapy. After total mesorectal excision, optional adjuvant chemotherapy was offered as 4 cycles of FU-FA treatment (Mayo regimen) within the six weeks after surgery.
Pathological examination of the surgical specimens
Resection specimens were thoroughly sectioned and at least five sections were submitted per tumor (unless the primary was so small that fewer sections can be done) for microscopic examination. In cases of complete response, suspected areas of the resection specimen were examined entirely. In addition, a vigorous search of the mesorectum was performed to identify as many lymph nodes as possible and all identified lymph nodes were submitted entirely for microscopic evaluation. For the evaluation of tumor response, Dworak grading system (12) was used by two gastrointestinal pathologists at Istanbul Medical Faculty, Department of Gastrointestinal Pathology. Pathologic response was evaluated using Total Regression Score (TRG), where TRG IV indicates no viable cancer cells and TRG 0 indicates no downgrading. Acellular pools of residual mucin in specimens were considered to represent completely eradicated tumor.
Follow-up
Patients were followed up routinely at 3-month intervals for the first 2 years after the operation and at 6-month intervals during next years. A local recurrence was defined as a radiologically demonstrated or a biopsy proven tumor within pelvis or perineum. Disease-free survival (DFS) and overall survival (OSS) were defined as the time from initiation of chemotherapy to the first evidence of relapse or death.
Statistical analysis
Statistical analysis was performed using SPSS software version 16.0 (SPSS, Chicago, IL). Kaplan-Meier method was used to analyze survival and the differences in survival probabilities were assessed using log-rank test. Mann-Whitney U, chi-square and Fisher’s exact tests were used, where applicable. Statistical significance was set at 0.05.
Results
Patient characteristics
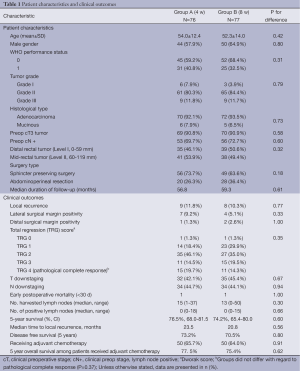
Between January 2002 and December 2007, 170 eligible patients were enrolled in the trial. Baseline characteristic and clinical outcomes of the patients are shown in Table 1. Groups were similar with regard to patient characteristics. Seventeen patients (9 patients from group A and 8 patients from group B) were withdrawn from the study due to following reasons: intestinal obstruction (2 patients), M1 identified at surgery (5 pts), patient preferred local excision (2 pts), frozen pelvis found at operation (5 pts), progression during treatment (3 pts).
Full table
Clinical outcomes
Lateral and distal surgical margin positivity was present in 11 and 3 patients, respectively. The two groups did not differ with regard to lateral surgical margin positivity and pathological tumor regression rate.
Local recurrence occurred in 9 (11.8%) and 8 (10, 3%) patients in Group A and Group B, respectively. Local recurrence rate was significantly higher among patients with surgical margin positivity (either lateral or distal) compared to patients with negative margins (28.5% vs. 9.3%, P=0.02).
Group A and Group B had similar 5-year overall survival (76.5% vs. 74.2%, P=0.60) (Figure 1A) and disease free survival rates (73.2% vs. 70.5%, P=0.80) (Figure 1B). Overall survival was better in patients with negative surgical margins (78.8% vs. 53.0%, P=0.04) (Figure 1C). In addition, local recurrence was associated with worse survival (37.7% vs. 80.3%, PFigure 1D). However, greater pathological regression grade (TRG III-IV) did not provide any overall survival benefit (Figure 1E). Similarly, receiving adjuvant treatment did not improve survival (77.4% vs. 75.4%, P=0.62).

Complications
Neoadjuvant chemoradiotherapy complications
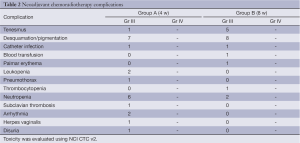
Twenty percent dose reduction in preoperative chemotherapy was required in 12 (15.7%) and 14 (18.8%) patients from Group A and Group B, respectively. Only two patients required interruption of radiotherapy (for one week). Neoadjuvant chemoradiotherapy related complications are listed in Table 2.
Full table
Adjuvant chemotherapy complications
Patients in 4-week group received 93.5% of planned postoperative chemotherapy cycles, whereas the corresponding figure was 92.5% in the 8-week group. Adjuvant chemotherapy grade III-IV complications were as follows: diarrhea, 7%; nausea/vomiting, 10%; stomatitis, 10%; leukopenia, 5%; decrease in Hb, 5%; angina, 2%; cerebrovascular accident, 1%; catheter infection, 2%; ileus, 2%. Dose reduction was required in 22% of the patients receiving adjuvant chemotherapy.
Surgical complications
There were two early postoperative deaths (one from each group). Surgical complications are shown in Table 3.

Full table
Long-term complications
In the long-term, renal complications due to local recurrences were seen in 7 patients (4.5%) and a nephrostomy tube was placed in all of them.
Discussion
This study was the first prospective randomized study conducted with rectum cancer patients to test the effect of the interval between preoperative neoadjuvant chemoradiotherapy and surgery on both pathological response to chemoradiotherapy and long-term outcomes including local recurrence and survival. A difference between long-interval (8 weeks) and short interval (4 weeks) groups could not be found in any of the parameters tested.
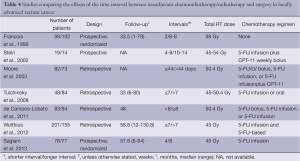
To date, several studies with varying methodology and sample size have examined the effects of neoadjuvant radiotherapy/chemoradiotherapy-surgery interval on treatment outcomes (Table 4) (6,8-10). An important issue to address is to show whether delaying surgery results in better pathological response in the tumor bed where most relapses occur. The second important question to answer is whether potential benefits of delaying surgery results in low recurrence rates or long-term survival gain. Studies that have examined these hypotheses are relatively scarce in number with conflicting results.
Full table
The earliest study comparing the effects of short interval (within 2 weeks) and long interval (6 to 8 weeks) between neoadjuvant therapy and surgery was the Lyon R90-01 randomized trial, which demonstrated better clinical and pathological response with longer interval (9). However, the interval did not have an effect on morbidity, local relapse, short-term survival and sphincter preservation. That study did not include a preoperative chemotherapy regimen. In 2002 and 2003, two studies with no long-term recurrence and survival data were published (6,10). These two studies, a prospective study by Stein et al. (10) and a retrospective study by Moore et al. (6), failed to demonstrate any benefit of long-term delay before surgery in terms of tumor downstaging, pathological response or sphincter preservation. A recent retrospective study by Tulchinsky et al. (8) examined both short and long-term results and found better pathological complete response, metastasis and disease free survival rates but similar overall survival and local recurrence. In line with most but not all of the findings of previous studies, the present prospective randomized study did not find any difference between surgery performed 4 weeks after neoadjuvant therapy and surgery performed after 8 weeks of delay, in terms of both early benefits of neoadjuvant therapy and long-term success of combined treatment. Two groups had similar pathological complete response, T and N downgrading, lateral surgical margin positivity, sphincter preservation rates as well as similar local recurrence, distant metastasis and 5-year survival rates.
Lateral or circumferential resection margin (CRM) has been shown to be the most important factor for the risk of local recurrence after rectal cancer surgery (13). The relevance of a positive CRM has been confirmed in many subsequent studies not only for local recurrence, but also for systemic failure (14-16). All studies that included the development of distant metastases as a separate outcome variable show a significant difference in prognosis between the CRM-positive and the CRM-negative patients (HR, 2.8; 95% CI, 1.9 to 4.3) regardless of the use of neoadjuvant therapy (11). In combination with lymph node status, CRM status seems to provide a better prognostic model than current TNM system (13). In the study by Bujka et al., the addition of fluorouracil/folinic acid to long-term radiotherapy did not decrease the number of positive margins although there was more downstaging in the radiochemotherapy arm (16). Similarly, more downstaging was present in the radiochemotherapy arm compared with the radiotherapy arm in the European Organization for Research and Treatment of Cancer trial but CRM positivity rate was similar. Thus, downstaging does not necessarily translate into CRM negativity, probably not into better long-term results in term of local recurrence and survival. This study used also lateral surgical margin positivity as an indicator of pathological response and did not find a significant difference between the short-interval and long-interval groups. This is consistent with the long-term results of this study demonstrating similarity in terms of local and systemic long-term results.
Among several studies that compared short versus long delay after neoadjuvant chemoradiotherapy in rectal cancer, only the study by Tulchinsky et al. showed significant benefits of delaying surgery in terms of pathological complete response and disease free survival, but not for overall survival (8). In that study, surgical margin positivity was not reported and the significance of the difference between disease free survival rates was only marginal. Similarly, in other two studies surgical margin positivity was not reported (17,18). Delayed surgery was associated with improved 3-year local recurrence rate (17) and increased complete pathological response rate (18) in the first and second study, respectively. The other two studies (6,10), one of which also examined surgical margin positivity (6), failed to show any difference between groups. Moreover, none of the studies other than the present study was randomized.
Previous relevant studies used different preoperative chemotherapy regimens and groups were heterogeneous, particularly in terms of the route and type of chemotherapy, raising the issue of potential bias. Study by Moore et al. used both oral and infusional forms of 5-FU (6). In that study, the use of infusional 5-FU was slightly more frequent in the long-interval group, although the difference was not statistically significant. In the study by Tulchinsky et al. (8), information on the homogeneity of the groups with regard to chemotherapy regimen was not provided. Difference in chemotherapy regimens may result in differences in both short- and long-term benefits. For example, Mohiuddin et al. has demonstrated that infusional 5-FU was associated with better outcomes than bolus administration of 5-FU (19). This study on the other hand, used a standard chemotherapy regimen in a prospective randomized design. In addition, distribution of the groups with regard to tumor distance from anal verge is an important parameter since low rectal tumors may be associated with higher local recurrence rate. The distribution of tumor distance was also homogenous in this study.
One of the concerns related to the prolongation of chemoradiotherapy-surgery interval is the potential of complications during or after surgery due to radiotherapy-induced fibrosis. In USA, surgeons prefer to perform surgery 4 to 8 weeks after neoadjuvant therapy (6). In a study by Tran et al., safety of prolonged interval after neoadjuvant treatment was examined (7). Although the sample size was relatively small in that study, surgical complication rates including intraoperative blood loss, postoperative complications and re-admissions were similar in patients operated after >8 weeks and <8 weeks of delay. However, length of operation and length of hospital stay were longer among patients operated >8 weeks after neoadjuvant treatment. The three studies, which were mainly designed for examining the efficacy outcomes of prolonging neoadjuvant-surgery interval, also found similar rates of blood loss, postoperative morbidity, and postoperative complications with longer intervals when compared to shorter intervals, although a slightly higher rate of anastomotic complications was observed in the study by Moore et al. (6). Unlike the findings of Tran et al., Moore and Tulchinsky failed to find a difference in terms of duration of operation and hospital stay (6,8). In this study, overall rate of postoperative complications was slightly higher in the patients that received surgery after a short delay. However, rates of individual postoperative complications, i.e., deep venous thrombosis, Fournier gangrene, and pneumonia, were similar.
Although current evidence suggest that delaying the operation seems safe in terms of intra- and postoperative complications, there is still concern that the tumor might progress or metastasize during the prolonged interval between neoadjuvant treatment and surgery, which was supported by the increased number of patients with ‘tumor upstaging’ in patients that received delayed surgery (7).
Conclusions
Findings of the present study do not support the intentional prolongation of the chemoradiotherapy-surgery interval in an effort to improve pathological response to radiochemotherapy, local disease control or survival; although prolonging the interval seems safe based on evidence from relatively low number of patients. Surgical margin positivity and quality of surgical performance seem to be more important.
Acknowledgements
This study has been presented at the general poster session of American Society of Clinical Oncology Annual Meeting, in Orlando, FL, May 29- June 2, 2009 (Abstract No, 4131).
Disclosure: The authors declare no conflict of interest.
References
- Gunderson LL, Sosin H. Areas of failure found at reoperation (second or symptomatic look) following “curative surgery” for adenocarcinoma of the rectum. Clinicopathologic correlation and implications for adjuvant therapy. Cancer 1974;34:1278-92. [PubMed]
- Gérard A, Buyse M, Nordlinger B, et al. Preoperative radiotherapy as adjuvant treatment in rectal cancer. Final results of a randomized study of the European Organization for Research and Treatment of Cancer (EORTC). Ann Surg 1988;208:606-14. [PubMed]
- Bosset JF, Collette L, Calais G, et al. Chemotherapy with preoperative radiotherapy in rectal cancer. N Engl J Med 2006;355:1114-23. [PubMed]
- Roh MS, Colangelo LH, O’Connell MJ, et al. Preoperative multimodality therapy improves disease-free survival in patients with carcinoma of the rectum: NSABP R-03. J Clin Oncol 2009;27:5124-30. [PubMed]
- Sauer R, Fietkau R, Wittekind C, et al. Adjuvant vs. neoadjuvant radiochemotherapy for locally advanced rectal cancer: the German trial CAO/ARO/AIO-94. Colorectal Dis 2003;5:406-15. [PubMed]
- Moore HG, Gittleman AE, Minsky BD, et al. Rate of pathologic complete response with increased interval between preoperative combined modality therapy and rectal cancer resection. Dis Colon Rectum 2004;47:279-86. [PubMed]
- Tran CL, Udani S, Holt A, et al. Evaluation of safety of increased time interval between chemoradiation and resection for rectal cancer. Am J Surg 2006;192:873-7. [PubMed]
- Tulchinsky H, Shmueli E, Figer A, et al. An interval >7 weeks between neoadjuvant therapy and surgery improves pathologic complete response and disease-free survival in patients with locally advanced rectal cancer. Ann Surg Oncol 2008;15:2661-7. [PubMed]
- Francois Y, Nemoz CJ, Baulieux J, et al. Influence of the interval between preoperative radiation therapy and surgery on downstaging and on the rate of sphincter-sparing surgery for rectal cancer: the Lyon R90-01 randomized trial. J Clin Oncol 1999;17:2396. [PubMed]
- Stein DE, Mahmoud NN, Anne PR, et al. Longer time interval between completion of neoadjuvant chemoradiation and surgical resection does not improve downstaging of rectal carcinoma. Dis Colon Rectum 2003;46:448-53. [PubMed]
- Nagtegaal ID, Quirke P. What is the role for the circumferential margin in the modern treatment of rectal cancer? J Clin Oncol 2008;26:303-12. [PubMed]
- Dworak O, Keilholz L, Hoffmann A. Pathological features of rectal cancer after preoperative radiochemotherapy. Int J Colorectal Dis 1997;12:19-23. [PubMed]
- Quirke P, Durdey P, Dixon MF, et al. Local recurrence of rectal adenocarcinoma due to inadequate surgical resection. Histopathological study of lateral tumour spread and surgical excision. Lancet 1986;2:996-9. [PubMed]
- Marijnen CA, Nagtegaal ID, Kapiteijn E, et al. Radiotherapy does not compensate for positive resection margins in rectal cancer patients: report of a multicenter randomized trial. Int J Radiat Oncol Biol Phys 2003;55:1311-20. [PubMed]
- Marijnen CA, Nagtegaal ID, Klein Kranenbarg E, et al. No downstaging after short-term preoperative radiotherapy in rectal cancer patients. J Clin Oncol 2001;19:1976-84. [PubMed]
- Bujko K, Nowacki MP, Nasierowska-Guttmejer A, et al. Sphincter preservation following preoperative radiotherapy for rectal cancer: report of a randomised trial comparing short-term radiotherapy vs. conventionally fractionated radiochemotherapy. Radiother Oncol 2004;72:15-24. [PubMed]
- de Campos-Lobato LF, Geisler DP, da Luz Moreira A, et al. Neoadjuvant therapy for rectal cancer: the impact of longer interval between chemoradiation and surgery. J Gastrointest Surg 2011;15:444-50. [PubMed]
- Wolthuis AM, Penninckx F, Haustermans K, et al. Impact of interval between neoadjuvant chemoradiotherapy and TME for locally advanced rectal cancer on pathologic response and oncologic outcome. Ann Surg Oncol 2012;19:2833-41. [PubMed]
- Mohiuddin M, Regine WF, John WJ, et al. Preoperative chemoradiation in fixed distal rectal cancer: dose time factors for pathological complete response. Int J Radiat Oncol Biol Phys 2000;46:883-8. [PubMed]


