Do all locally advanced rectal cancers require radiation? A review of literature in the modern era
Department of Radiation Oncology, University of Arizona, Tucson 85724, Arizona, USA
|
Review Article
Do all locally advanced rectal cancers require radiation? A review of literature in the modern era
Department of Radiation Oncology, University of Arizona, Tucson 85724, Arizona, USA
|
|
Abstract
Potentially curable rectal cancer is primarily treated with surgical resection. Adjuvant or neoadjuvant radiotherapy is often
utilized for patients deemed to be at unacceptable risk for local recurrence. The purpose of this article is to review the pertinent
literature and elucidate the role of radiotherapy in patients with an intermediate risk of local recurrence. The addition
of chemoradiotherapy is recommended in the majority of patients with transmural or node positive rectal cancer. However,
some patients with favorable characteristics may have only a small incremental benefit from the addition of radiotherapy. The
decision to treat or not to treat should take into consideration the patient and physician tolerance of risk of recurrence and risk
of treatment related toxicity. The primary factors identified for determining low risk patients are circumferential radial margin
(CRM), location within the rectum, and nodal status. Patients at lowest risk have widely negative CRM (>2mm), proximal lesions
(>10cm from the anal verge), and no nodal disease. Patients with all three low risk factors have an absolute reduction in
local recurrence that is <5% and may be eligible to forego radiotherapy. Additional factors identified which may impact local
recurrence risk are elevated serum CEA level, lymphovascular space invasion, pathologic grade, and extramural space invasion.
Key words combined modality therapy; rectal cancer; neoadjuvant chemoradiation; adjuvant therapy; radiation thera
J Gastrointest Oncol 2010; 1: 45-54. DOI: 10.3978/j.issn.2078-6891.2010.008
|
|
Introduction
The addition of radiotherapy to surgery for locally advanced
rectal cancer has demonstrated improvement in local control
in historic randomized trials (1,2,3). An improvement
in overall survival has not been shown in the majority of
studies; only a single Swedish rectal cancer trial demonstrated
an improvement in overall survival with the addition of
short course neoadjuvant radiotherapy to surgery (4). This
landmark trial reported a local recurrence improvement from
27% to 11% with the addition of preoperative radiotherapy.
This translated into a survival benefit of 10% at 5 years
(48% vs. 58%). While the majority of randomized data has
not corroborated this survival benefit, the morbidity of local recurrence and relatively poor salvage rates have been
sufficient to justify radiotherapy as standard practice for
stage II or III rectal cancer. Nonetheless, there are subsets
of patients with stage II or III disease who are expected
to have low absolute benefit from radiation therapy, and
the therapeutic ratio may be insufficient to routinely
recommend radiation. Furthermore, advances in surgery and
chemotherapy have called into question the role of radiation
in the modern treatment era. This review is to discusses
factors that should be considered when determining which
patients should receive adjuvant or neoadjuvant radiation
therapy.
|
|
Total mesorectal excision
The advent of the total mesorectal excision (TME), which
utilizes sharp dissection through a plane between the visceral
and parietal layers of the pelvic fascia to excise the tumor
and mesorectum en bloc, has dramatically improved local
control following surgery (5). TME mobilizes the rectum
from the sacral promontory to the pelvic floor, with a 5-6 cm
mesorectal margin distal to the lowest edge of the primary
tumor. Prior to TME, surgery was typically performed with
blunt dissection, without close attention to circumferential margin. Resection of the mesentery with its blood supply and
lymphatics maximizes the probability of clear circumferential
margins, and removes mesorectal lymph nodes at risk for
harboring metastatic disease. A review of the literature
encompassing more than 5000 patients reports local
recurrence rates of 6.6% with TME, compared to about 15%
in similarly staged patients treated without TME (6,7,8). The
success of TME is dependent on surgeon training, and rectal
cancer patients should be treated by surgeons experienced in
this technique (9,10).
While TME has decreased local recurrence, thus
decreasing the absolute benefit of radiotherapy, a randomized
trial by the Dutch demonstrated that the addition of radiation
to TME decreases local recurrence (11). In this trial 1861
stage I to III rectal cancer patients were randomized to TME
with or without short course neoadjuvant radiation therapy
(25 Gy in 5 fractions). Local relapse at 2 years was 2.4% in
patients who received radiation, versus 8.2% in those who
did not (p<0.001), with equivalent 2 year overall survival
rates of 82%. It should be noted, however, that this study
did not include chemotherapy, and therefore the benefit of
radiation added to chemotherapy remains a topic of debate.
As discussed in more detail below, the absolute benefit of
radiation is dependent on tumor characteristics including
circumferential margin, location in the rectum, and stage. be treated by surgeons experienced in
this technique (9,10).
Influence of circumferential radial margin
Prior to the development of TME, it was recognized that
circumferential radial margin (CRM) had a dominant
influence on local relapse. In the landmark study by Quirke et
al., rigorous pathologic analysis revealed 27% occult positive
CRM after potentially curative surgery (12). This correlated
with a 23% local failure rate. Subset analysis of Dukes’ B
patients revealed 5% CRM involvement and a subsequent
local failure rate of 5%. A subset analysis of the Swedish
rectal cancer trial examined local failure after curative or
noncurative surgery (13). The authors did not differentiate
noncurative resection due to proximal, distal, or radial margin
status. Local failures were much more common in patients
who received a noncurative resection (34% vs. 16%). The
addition of preoperative radiation improved local control
for patients with curative resection (24% vs. 9%) as well as
noncurative resection (44% vs. 23%).
Following the advent of TME, local recurrences were
reduced, in part due to wider CRM. Nonetheless, close or
positive CRM remains a predictor of local recurrence. A
retrospective analysis of the influence of CRM status on
local control in the aforementioned Dutch preoperative
radiotherapy trial was reported by Nagtegaal et al (14).
In non-irradiated patients, tumor involving the surgical margin or within 2 mm of the surgical margin resulted in 2
year local recurrence rates of 16.4% and 14.9% respectively
(non-significant difference). However, a surgical margin >2
mm resulted in a 2 year local failure rate if 5.8% (p=0.0007
compared to CRM <2mm). The authors further subdivided
width of CRM to show that the benefit of increased margin
continued beyond 2 mm. Surgical margins of 2-5 mm, 5-10
mm, and >10 mm resulted in local recurrence rates of 10.3%,
6.0%, and 2.4% respectively. In this study, location within
the rectum and TNM stage strongly affected the likelihood
of obtaining a negative CRM. Distal lesions (<5 cm from
the anal verge) had involved margins in 25.9% of patients,
compared to only 13.2% and 16.5% for lesions 5-10 cm and
10-15 cm from the anal verge, respectively (p=0.009 for
trend). In regards to stage, positive margins were noted in
2.0%, 14.6%, and 33.1% of patients with stage I, II, and III
disease, respectively (p<0.001 for trend). Due to the low
rate of local recurrence in patients with stage I or II disease,
circumferential margin was no longer of predictive value for
local failure.
The Medical Research Counsel examined the use of short
course preoperative radiotherapy versus selective adjuvant
chemoradiation therapy in patients with close CRM in a
prospective randomized trial, MRC CR07 (15). All patients
underwent TME. One arm received neoadjuvant short
course radiotherapy, consisting of 25 Gy in 5 fractions. The
second arm received upfront TME, and patients who were
found to have CRM closer than 1mm were treated with
chemoradiotherapy consisting of 45 Gy in 25 fractions with
concurrent 5-fluorouracil. No radiation was given if CRM was
>1mm. Adjuvant chemotherapy was given to patients in either
arm as per the standards of the treating institution (declared
prospectively). A total of 1350 patients were enrolled. The
short course of preoperative radiotherapy did not have a
discernable downstaging affect on margin status (positive
margin rate 10% with preoperative radiotherapy vs. 12%
with upfront surgery), likely due to the short delay between
starting RT and surgery (7 days), which was insufficient to
allow for significant tumor shrinkage. However, preoperative
radiotherapy provided a significant improvement in local
recurrence (4.4% vs. 10.6% at 3 years, p<0.0001) and diseasefree
survival (77.5% vs. 71.5% at 3 years, p=0.013). The
authors suggest that while margin status is a strong predictor
of local recurrence, selective adjuvant chemoradiation therapy
for close margins is inferior to preoperative radiotherapy
in terms of local control and disease free survival. In other
words, radiation provides a benefit even in patients with
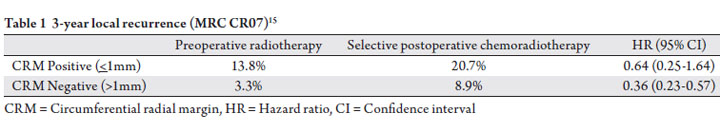
CRM >1mm (Table 1).
In a separate analysis of 1156 in the MRC CR07 trial who
had detailed pathological data available, the authors showed
that the plane of surgery (mesorectal, intramesorectal, or muscularis propria plane) influenced local control, with 3
year local recurrence rates of 4%, 7%, and 13%, respectively
(p=0.0011) (16). Although plane of surgery was an
independent predictor of local recurrence, there was no
evidence that the benefit of radiation was dependent on the
plane of surgery (p=0.3 for trend). The effects of optimal
mesorectal resection and radiation were additive, with 3 year
local recurrence rate of 1% in patients who had short course
preoperative radiotherapy and mesorectal plane of resection.
Radiation reduced local recurrence by greater than 50%
regardless of plane of resection.
CRM status remains an important indicator of local
control in the era of TME, as recognized in NCI consensus
guidelines (17). CRM of >2mm is preferable, though the risk
of recurrence is likely a continuum, with larger margins at
lower risk of recurrence. The presence of close CRM is one
factor influencing the decision of whether or not to employ
adjuvant radiation therapy, though the MRC CR07 trial
suggests that radiation decreases local recurrence even in
the setting of CRM >1mm (Table 1). Part of the challenge
for treating physicians is deciding on whether the degree of
benefit of local control justifies the potential toxicities, and
the decision to use radiation will depend on a constellation of
risk factors rather than margin status alone.
MRI scan has been used as a tool to predict negative
circumferential margin, with a meta-analysis reporting
sensitivity of 94% and specificity of 85% (18). The use of MRI
scan to identify patients more likely to benefit from radiation
therapy, however, remains investigational.
 Location
The anatomic definition of the proximal extent of the rectum
is debated. The rectum is extraperitoneal on its posterior
surface. The upper one-third of the rectum is covered by
the peritoneum on the anterior and lateral surfaces, and the
inferior two-thirds of the rectum is completely extraperitoneal.
The proximal extent of the rectum has classically been
defined as the peritoneal reflection. The peritoneal reflection
cannot be visualized by imaging studies. Rather, it is defined
at the time of operation. Therefore, whether or not a tumor
is in the true rectum can be challenging to determine
prior to surgery. In the adjuvant setting, randomized trials
demonstrating a benefit to radiation in stage II or III disease have variably defined the rectum as below the peritoneal
reflection, below the sacral promontory, <12 cm from the
anal verge on rigid proctoscopy, or <16 cm from the anal
verge (1,2,15,19,20,21,22). Neoadjuvant trials do not allow
for intraoperative evaluation of the peritoneal reflection, and
have variably included patients with tumor from <12 cm to
<16 cm from the anal verge (15,21). Yun et al. reported that
the average length of the posterior peritoneal reflection from
the anal verge at the time of surgery was 14 cm in 46 patients,
and it correlated with patient height (23). Whether or not the
tumor lies within the rectum influences treatment decisions
as colon cancer has no proven benefit from radiation therapy,
and making this determination prior to surgery remains a
challenge for physicians.
Even if the tumor lies within the rectum, proximal rectal
cancers have relatively lower benefit from radiation compared
to distal. Prior to the advent of TME, the MRC working
group identified location in the rectum as a prognostic factor
in a randomized trial of preoperative radiotherapy (3).
Lesions less than 8 cm from the anal verge had a 5 year local
disease free survival rate of 52%, vs. 62% for lesions greater
than 8 cm from the anal verge (p=0.008). This difference
translated into an overall survival difference at 5 years of
35% for distal lesions compared to 48% for more proximal
lesions (p<0.001). While distal tumors may represent a more
challenging surgery, this trial showed no difference in the rate
of gross total resection as assessed by the surgeon (62% with
distal lesions and 65% with proximal lesions). Circumferential
margins status, however, was not assessed.
Despite reductions in local recurrence in the TME era,
distal lesions continue to carry a worse prognosis. The Dutch
rectal cancer trial reported that increasing distance from
the anal verge was associated with higher local control on
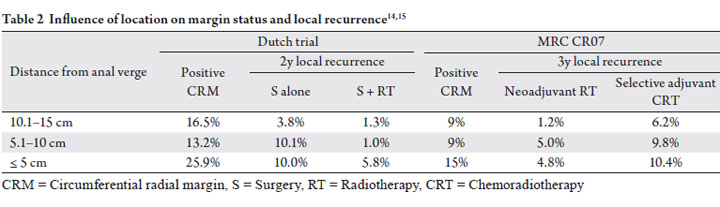
multivariate analysis (p=0.02, Table 2) (11). On univariate
analysis, the addition of radiation therapy to TME did not
improve local control in tumors more than 10 cm from the
anal verge. Multivariate tests, however, suggested that the
favorable effects of radiation probably didn’t differ based on
location in the rectum. This trial was not adequately powered
to determine whether or not radiation has a small impact on
local control in the proximal rectum, but at a minimum this
trial demonstrates that the absolute benefit of radiation in the
proximal rectum, if present, is small.
The Dutch trial revealed an increased incidence of positive margins in distal tumors within 5cm from the anal verge
(Table 2) (14). Interestingly, lesions located between 5
and 10 cm from the anal verge had an incidence of positive
margins similar to more proximal lesions but an intermediate
local failure rate. This suggests that margin status alone is not
sufficient for predicting local recurrence and tumor location
is an important independent consideration.
Similar to the results of the Dutch trial, the MRC CR07
trial comparing preoperative radiotherapy to selective
adjuvant chemoradiotherapy demonstrated that tumor
location influences local recurrence and CRM positivity
(Table 2) (15). CRM was positive in 15% of patients with
distal extent of tumor 0-5cm from the anal verge, versus 9%
of patients with distal extend of tumor >10 cm from the anal
verge (p=0.004) (16). Neoadjuvant radiotherapy was found
to be superior to selective adjuvant chemoradiotherapy for
all tumor locations (Table 2). Although local recurrence rates
were higher with mid/distal disease compared to proximal
disease, the absolute benefit in 3-yr local control with the
addition of radiation was about 5%, regardless of location in
the rectum.
In summary, the Dutch study suggests proximal tumors
likely have a lower absolute benefit in local control from the
addition of radiation to surgery, while the MRC trial does
not, despite showing that distal tumors are more likely to have
positive CRM. Unfortunately, both trials include stage I to III disease, and neither trial addresses the benefit of radiation
based both on T stage and location. Specifically, the benefits
of radiation in T3N0 proximal disease are of interest. Further
study is needed to validate or refute the role of radiation in
proximal T3N0 disease.
 Influence of nodal status
As one would expect, the presence of malignant disease
within regional lymph nodes increases the risk of localregional
recurrence. Stocchi et al. retrospectively reviewed
patients enrolled in 3 North Central Cancer Tumor Group
(NCCTG) trials, and confirmed the prognostic value of nodal
status on local-regional recurrence (24). Eligible patients had
either T3-4 or N+ disease without distant metastases. Fiveyear
local-regional failure rates for patients with T3 disease
were 10%, 15%, and 32% for N0, N1, and N2, respectively.
Gunderson et al. expanded the Stocchi analysis to include
patients enrolled in NSABP R01 and R02 trials, for a total of
3791 evaluable patients (25). Again nodal involvement was
predictive of local failure with recurrence rates of 9%, 11%,
and 13% for N0, N1, and N2 disease, respectively (p=0.005).
These authors evaluated outcomes with surgery alone,
surgery plus chemotherapy, and surgery plus chemoradiation
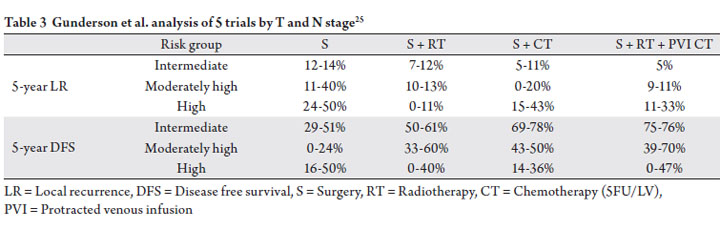
based on T stage and N stage (Table 3). Given the relatively
low number of patients in certain subsets and given the retrospective nature of this study, the value of the addition
of radiation to surgery and chemotherapy could not be
answered. Nonetheless, the authors identified an intermediate
risk group (T3N0, T1-2N1), a high intermediate risk group
(T1-2N2, T3N1, T4N0), and a high risk group (T3-4N2,
T4N1), and suggest that the intermediate risk group is the
least likely to benefit from the addition of radiation therapy
to chemotherapy. The studies included in this analysis were
completed prior to the advent of TME and prior to the
adoption of newer chemotherapies including oxaliplatin,
and irinotecan. Furthermore, some utilized bolus rather than
protracted venous 5FU, the latter of which has demonstrated
superiority in a randomized trial (22). Therefore, the results
of this study, while intriguing, are not directly applicable to
the modern era. The use of TME and modern chemotherapy
may further decrease the relative benefits of radiation,
particularly in the intermediate risk group.
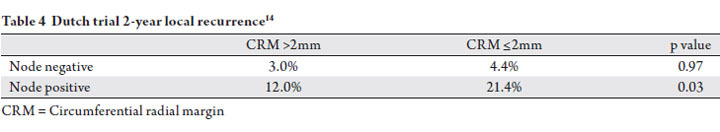
The Dutch trial demonstrated a connection between nodal
status and CRM status after TME in the 769 patients who
did not receive radiation (14). Patients with stage II disease
(T3-4 N0) had a 14.6% rate of positive CRM (≤1mm),
compared to 33.1% for patients with Stage III disease (T1-4
N1). This increase in positive CRM is due to the correlation
of nodal disease with more advanced primary tumors as
well as the physical presence of malignant lymph nodes near
the resection margin. Nodal disease determined the closest
margin in 24.9% of patients with nodal disease. Interestingly,
the predictive value of margin status was dependent upon
whether the margin was determined by the primary tumor or
lymph node. The 2-year local failure rate for stage III patients
was reported as 22.1%, 12.4%, and 12.0% for positive margin
by primary tumor, positive margin by lymph node, and >2mm
negative margin, respectively. This indicates that the presence
of nodal disease at the margin does not worsen the prognosis
for node positive patients. Additionally, the authors identified
that nodal status predicted for local failure independent of surgical margin (Table 4). This analysis further supports
the role of radiation in node positive disease, particularly in
patients with positive margins. As previously discussed, this
study did not include chemotherapy, and therefore the benefit
of radiation added to chemotherapy remains a topic of debate.
The MRC CR07 of short course preoperative radiation
therapy versus selective postoperative chemoradiotherapy in
patients with close CRM similarly reported that the subset of
patients with node positive disease (stage III) had higher local
recurrence rates compared to stage I or II on multivariate
analysis (p<0.0001), and also had a greater absolute reduction
in local recurrence with the use of neoadjuvant radiation
(15,16). Three year local recurrence rate was 7.4% in node
positive patients treated with neoadjuvant radiotherapy
versus 15.4% in node positive patients treated with selective
adjuvant chemoradiotherapy. Three year local recurrence
rate was 1.9% in stage II patients treated with neoadjuvant
radiotherapy versus 6.4% in stage II (node negative) patients
treated with selective adjuvant chemoradiotherapy (Table
5). Only 12% of patients enrolled in the selective adjuvant
chemoradiation arm of the study had positive circumferential
margins. Therefore, the majority of patients in this arm of
the study did not receive radiotherapy, and the trial is largely
comparing neoadjuvant radiation versus no radiation. The
results of this study suggest that patients with clinically
apparent nodal disease benefit from radiotherapy and in
particular from neoadjuvant radiotherapy.
   Influence of chemotherapy
While local recurrence represents a morbid event, distant
disease remains the primary obstacle to cure, and the majority
of recurrences are distant. Systemic therapy in locally
advanced disease decreases distant metastases and improves
survival. Adjuvant chemotherapy in the absence of radiation
has not, however, been shown to improve local control. Trials addressing this issue accrued patients during the pre-TME
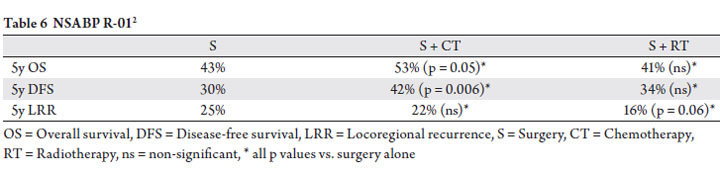
era. The NSABP R-01 trial compared observation vs. adjuvant
radiotherapy vs. adjuvant chemotherapy (fluorouracil,
semustine, and vincristine) (2). The authors described an
improvement in the 5-year disease-free survival and overall
survival in the chemotherapy arm vs. observation arm, but
not local control (Table 6).
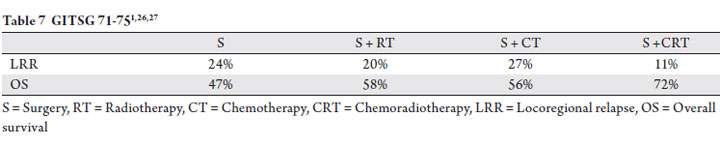
Similarly, a prospective trial by the Gastrointestinal Tumor
Study Group did not show a decrease in local control with
the addition of chemotherapy alone to surgery. This trial
randomized patients to surgery followed by observation,
chemotherapy, radiotherapy or chemoradiotherapy (1,26,27).
The trial was closed early due to inferiority of the surgery
alone arm and thus the data was not sufficiently powered to
distinguish outcomes all four treatment arms. At a median of
80 months, the locoregional recurrence and overall survival
were improved by adjuvant chemoradiotherapy, but not by
either therapy alone (Table 7).
Randomized trials showed that the addition of radiation
to chemotherapy improved local control in the pre-TME era,
but the benefit of adding radiation to modern chemotherapy
following TME is not known (1,19,26). The Dutch
study of TME with or without short course preoperative
radiation therapy proved that the addition of radiation
to TME improves local control, but this trial did not use
chemotherapy. It is possible, though not proven, that the
lower disease burden afforded by modern surgical techniques
may be amenable to local control with chemotherapy,
particularly with the use of newer, more active chemotherapy
regimens. These advances may obviate the benefit of adjuvant
radiotherapy in some patients.
The most notable advances in chemotherapy for rectal
cancer are oxaliplatin and irinotecan. Oxaliplatin is a platinum derivative that acts as an alkylating agent and impairs DNA
replication and transcription. A randomized trial by de
Gramont et al. showed improvement in response rate in
advanced colorectal cancer from 22% with infusional 5FU
plus leucovorin to 50.7% with infusional 5FU, leucovorin,
and oxaliplatin (FOLFOX), p=0.0001 (28). Irinotecan is a
topoisomerase I inhibitor. A randomized trial by Douillard
et al. showed improvement in response rate in advanced
colorectal cancer from 22% with infusional 5FU plus
leucovorin to 35% with infusional 5FU, leucovorin, and
irinotecan (FOLFIRI), p<0.005 (29). While response rates
are higher with the addition of newer agents to 5FU, it is
unknown of these agents can provide equivalent local control
compared to radiation.
Biologic agents including bevacizumab, cetuximab, and
panitumumab have improved response rates, though these
improvements in response rates have had a relatively small
impact on survival in the metastatic setting, and to date have
no proven benefit in terms of survival in the adjuvant setting
(30). Bevacizumab is an antiangiogenic monoclonal antibody.
Cetuximab and panitumumab are monoclonal antibodies
directed against EGFR. KRAS mutation status is a strong
predictor of response to EGFR inhibitors, and on-going
studies are evaluating the benefit of cetuximab in KRAS wildtype
rectal cancer patients. These agents are not routinely
used in the adjuvant setting, and therefore at this time their
use does not impact radiation therapy recommendations. The
early results have been reported by Schrag et al. evaluating
6 cycles of induction FOLFOX-bevicizumab chemotherapy
without preoperative radiotherapy for patients with clinical
response (31). All 29 patients achieved clinical response
and proceeded to surgery with 8 patients (27%) achieving
a pathologic complete response. These results are certainly intriguing and we await the matururity and validation in
future trials.
  Other considerations
Other factors influencing the decision of whether or not
to utilize radiation may include CEA, lymphvascular space
invasion, grade, extramural vascular invasion, and distal
margin status. Nissan et al. reported on the experience
at Memorial Sloan Kettering of TME without adjuvant
therapy for pT2 (N=45) or early pT3 (N=49) well to
moderately differentiated tumors with negative lymph
nodes and a negative margins (32). The authors reported
a local recurrence rate of 10% at 8 years. Within this select
group of low risk patients, elevated CEA and the presence of
lymphvascular space invasion were associated with increased
risk of local recurrence. Patients with preoperative CEA
levels of ≥5 ng/mL had local recurrence rate of 21% at 8 years
vs. 0% in patients with CEA <5 ng/mL. The rate of pelvic
recurrence at 5 years was 32% vs. 6% with and without LVI,
respectively. No difference in local recurrence was found
based upon distal margin status more or less than 2 cm. Of
note, pelvic recurrence in this study was not influenced by
T stage, suggesting the T3N0 disease excised with negative
circumferential margins may be appropriately treated with
surgery alone. This study is limited, however, by a relatively
small number of patients. Furthermore, this study was a
retrospective analysis of a prospective database.
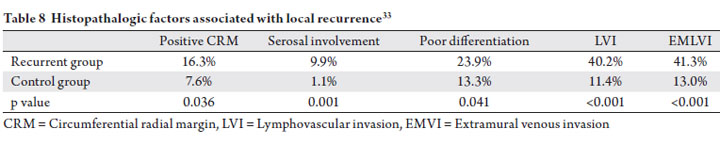
An analysis by Dresen et al. of Dutch patients who
developed isolated local failure also elucidates factors
correlated with recurrence in the TME era (33). Patients
who developed an isolated local recurrence were matched
with a control group who did not fail locally. All patients
were treated with TME with or without neoadjuvant therapy.
The authors reported positive CRM, serosal involvement,
poor differentiation, lymphovascular invasion (LVI), and
extramural venous invasion (EMVI) were all found more
frequently in the recurrent group, and were associated with
higher risk of local recurrence on multivariate analysis
(Table 8). While these findings need to be evaluated
prospectively, the identified histopathologic factors may
be used in conjunction with tumor stage, location, and nodal involvement to partition patients into risk groups for
consideration of adjuvant treatment.
 |
|
Neoadjvuant versus adjuvant radiation therapy
Neoadjuvant chemoradiation therapy has been shown to
be superior to adjuvant chemoradiation therapy in locally
advanced rectal cancer in a randomized study by the German
Rectal Cancer Group (21,34). Compared to adjuvant
chemoradiation, neoadjuvant chemotherapy decreased local
recurrence and decreased anastomotic stricture rates. This
improvement is in spite of the fact that patients randomized
to preoperative radiotherapy were more likely to have distal
lesions. This supports that for patients with clear indications
for radiation therapy, it is preferable to deliver therapy prior
to surgery. It is noteworthy, however, that 18% of patients in
this study who were clinical stage II or III who had immediate
surgery were found to be pathologic stage I, despite the use
of endoscopic ultrasound. Therefore, the use of preoperative
chemoradiation likely over-treats some patients. One
strategy is to treat patients with intermediate risk disease
(T3N0 proximal rectal cancer) with immediate surgery, and
deliver adjuvant radiation therapy if high risk features are
identified pathologically (T4, node positive, close/positive
margin). However, such an approach may result in the need
for adjuvant therapy in a significant proportion of patients.
Lombardi et al reported that in 32 patients with clinical
T3N0 low rectal cancer based on EUS, MRI, and PET/CT,
9 (28%) had pathologic node positive disease following
neoadjuvant chemoradiation. These patients would have been
under-treated with immediate surgery (35). In the absence
of randomized data evaluating the impact of radiation on
both disease control and quality of life specifically in the
T3N0 population, clinical judgement and patient education
regarding risks and benefits are essential.
Another consideration in choosing neoadjuvant versus
selective adjuvant radiation therapy includes whether or not
surgery will require abdominal perineal resection (APR) with
permanent colostomy. The German Rectal Cancer Study
group prospectively followed a subgroup of 188 patients
in whom the surgeon declared prior to randomization that
APR was required. In that subgroup, 19% who underwent neoadjuvant chemoradiation and 39% who underwent
adjuvant chemoradiation has sphincter sparing surgery after
APR (p=0.004). Therefore, neoadjuvant radiation therapy
improved the likelihood of sphincter preservation. Despite
these findings, it remains controversial if the surgical plan
should be modified based on response to chemoradiation, as
there remains the possibility of microscopic disease beyond
the grossly visible disease. A prospective pathologic analysis
from investigators at Memorial Sloan Kettering Cancer
Center showed that intramural extension beyond the gross
mucosal edge of the residual tumor was observed in only
2 of 109 patients (1.8%), and in both of these patients the
intramucosal spread was <1 cm (36). Moore et al. did not
identify distal margin <1cm as a predictor of local recurrence
after neoadjuvant chemoradiation (37). Therefore, patients
with good response to neoadjuvant chemoradiation have the
possibility of enhanced sphincter preservation, and in patients
in whom the requirement of APR is equivocal, it is reasonable
to consider neoadjuvant therapy in an attempt to enhance
rates of sphincter preservation. It should be recognized,
however, that data supporting sphincter preservation
following chemoradiation in patients who would otherwise
require APR is based on relatively small numbers of patients,
and equivalence to APR in terms of local control has not
been proven in a randomized fashion. Furthermore, the fecal
continence rates following low anterior resection requiring
intersphincteric resection are likely inferior to conventional
coloanal anastomosis, and therefore decisions regarding
sphincter preserving surgery need to take into account
anticipated sphincter function and its impact on quality of life
(38).
Toxicity of radiation
The decision of whether or not to use radiation therapy is
dependent not only upon the anticipated benefits in local
control, but also upon potential toxicities. The authors
of the MRC CR07 completed prospective quality of life
questionnaires for patients who underwent short course
neoadjuvant radiation therapy versus selective postoperative
chemoradiation (39). As noted previously, only 12% of
patients in the selective postoperative chemoradiation group
underwent chemoradiation, and therefore this trial in large
part evaluates radiation versus no radiation in terms of
quality of life. There was no difference in physical function,
general health, or overall bowel problems between the 2
arms. However, more patients who received preoperative
radiation therapy reported “unintentional release of stools”
at 2 years (53% vs. 37%, p=0.007). It is noteworthy that the
bulk of patients reported only “a little” unintentional release
of stools (43% vs. 29%). Only a minority of patients report “very much” unintentional release of stool (3% vs. 2%). This
analysis also demonstrated that surgery impacted mean male
sexual function score by more than 30 percentage points; the
addition of neoadjuvant short course radiation to surgery
further worsened sexual function score by 8-10%. Therefore,
radiation impacted male sexual function, though not to as
great a degree as surgery. Reponses from women with regards
to sexual function were insufficient to analyze.
Long term follow-up of the Dutch study similarly showed
higher rates of fecal incontinence in patients who received
short course preoperative radiation compared to those who
did not receive radiation (62% versus 38%, p<0.001) and
higher rates of anal blood loss (11% versus 3%, p<0.004).
There were no differences in hospitalizations or urinary
function. Furthermore, overall perceived health did not differ
in patients who did or did not receive radiation (p=0.38)
(40). The Swedish prospective randomized of short course
preoperative radiation therapy also demonstrated a small but
tangible risk of radiation induced malignancy exists (relative
risk 1.8 compared to no radiation) (41).
Currently in the United States, long course chemoradiation
(about 45-50 Gy in 1.8-2 Gy fractions) is typically used
rather than short course radiation. Haddock et al. reported
slight worsening of bowel function one year after long
course chemoradiation compared to baseline (median bowel
movement frequency increased from 1 to 2, with increased
urgency, clustering, and continence scores persistent one year
after therapy). Despite worsened continence scores, the need
for protective clothing did not increase above baseline (42).
Other prospective trials using long course chemoradiation
report severe (grade 3 or higher) late gastrointestinal toxic
effects in 2-15% of patients (21,43). Stricture at the anastomic
site occurs in 4-12% of patients, with lower likelihood if
radiation is delivered preoperatively (21). Severe late bladder
toxicity occurs in less than 1-4% of patients, and femoral head
fractures occur in less than 1% (21,43).
In summary, radiation therapy is associated with increased
incidence of late side effects, most commonly gastrointestinal.
Further study is needed to determine the degree to which
these side effects impact quality of life, and the risk of side
effects needs to be balanced with the expected improvements
in local control.
|
|
Conclusion
Neoadjuvant chemoradiotherapy is recommended in the
majority of patients with transmural or node positive rectal
cancer. However, some patients are in a favorable subgroup
in which the incremental benefit of radiotherapy
may be small. Factors to consider are proximal location
(>8-10 cm from the anal verge), negative margins (>1-2 mm), and absence of nodal disease. Additional factors
including low preoperative CEA (<5 ng/mL) and absence
of lymphovascular space invasion have been reported
as risk factors for local recurrence, though their use in
deciding whether or not to use radiation require validation
in prospective studies. Randomized data from the MRC
CR07 study and the Dutch study both show that the addition
of radiation to TME improves local control. However, in
patients with proximal location, negative circumferential
margins, and node negative disease, the absolute reduction
in local recurrence is <5%. This raises the possibility that
patients with proximal, T3N0 lesions with negative CRM
may represent an extremely favorable subgroup eligible to
forego neoadjuvant radiotherapy and instead receive adjuvant
radiation only in the setting of positive margins or surgical upstaging.
Since neoadjuvant radiotherapy appears to provide
some local control benefit in all subgroups of stage II and
III rectal cancer, the decision to treat or not to treat should
take into consideration the patient and physician tolerance
of risk of recurrence and risk of treatment related toxicity.
Prospective studies are warranted to determine if subgroups
of patients, such as T3N0 proximal disease, do not require
radiation therapy.
|
|
References
Cite this article as:
Vonk D, Hazard L. Do all locally advanced rectal cancers require radiation? A review of literature in the modern era. J Gastrointest Oncol. 2010;1(1):45-54. DOI:10.3978/j.issn.2078-6891.2010.008
|