The aspartate aminotransferase to platelet ratio before chemotherapy predicts adverse events for FOLFOX and XELOX regimens including bevacizumab as the first-line therapy for stage IV, recurrent and metastatic colorectal cancer
Introduction
Oxaliplatin and 5-fluorouracil/leucovorin (FOLFOX), with or without bevacizumab (BEV), has been shown to improve the response rates, progression-free survival, and overall survival in patients with stage IV or recurrent colorectal cancer (1,2). Capecitabine combined with oxaliplatin (XELOX) has also been shown to be non-inferior to FOLFOX4 as the first line treatment for patients with metastatic colorectal cancer (NO16966) (3). In patients with colorectal liver metastases, survival benefits were suggested when these regimens were used in a neoadjuvant or conversion setting before hepatectomy (4-6). However, oxaliplatin-induced hepatotoxicity, i.e., sinusoidal obstruction syndrome (SOS) is now commonly recognized as an adverse event related to these treatments (7), and must be carefully considered due to its association with a higher incidence of postoperative complications, especially hepatic insufficiency, after a major hepatectomy (8,9). Among the factors predicting SOS after chemotherapy, oxaliplatin-induced splenomegaly (10) is considered to be important because the grade of splenomegaly is associated with the severity of oxaliplatin-induced SOS (11). In addition, the ratio of aspartate aminotransferase to platelets (APR), thus indicating liver fibrosis due to chronic hepatitis (12), has been shown to be a simple predictor of oxaliplatin-induced SOS (13).
Among the various predictors of oxaliplatin-induced SOS recognized after chemotherapy, no single factor can predict the development of adverse events before oxaliplatin-based chemotherapy, although a gene polymorphism has been shown to be associated with adverse events after use of the FOLFIRI regimen (14). This is important, because the choice of whether or not to perform preoperative chemotherapy for patients with initially resectable colorectal liver metastases could be made based on the likelihood of adverse events if a predictor of SOS could be identified. We recently reported that the APR before chemotherapy can predict oxaliplatin-induced splenomegaly and also indicate the likelihood of developing adverse events during oxaliplatin-based chemotherapy (15). However, bevacizumab (BEV), a therapeutic antibody used for various cancers, including colorectal cancer, was recently reported to reduce the oxaliplatin-induced splenomegaly (16). Therefore, the aim of the present study was to investigate whether the APR before chemotherapy can predict the development of splenomegaly and adverse events due to FOLFOX/BEV and XELOX/BEV in patients with stage IV or recurrent colorectal cancer.
Patients and methods
We retrospectively reviewed patients with stage IV or recurrent colorectal cancer treated in our department between June 2007 and December 2011. We focused on patients undergoing chemotherapy consisting of FOLFOX/BEV or XELOX/BEV. The FOLFOX consisted of a 2 hrs intravenous infusion of oxaliplatin (85 mg/m2) with leucovorin (200 mg/m2), followed by a fluorouracil (400 mg/m2) intravenous bolus injection and a 46 h continuous infusion of fluorouracil (2,400 mg/m2) every two weeks. XELOX consisted of a 2 h intravenous infusion of oxaliplatin (130 mg/m2) on day 1 plus p.o. capecitabine (1,000 mg/m2) twice daily on days 1-15 of a 3-week cycle. BEV at a dose of 5 mg/kg with FOLFOX or 7.5 mg/kg with XELOX was administered as a 30 to 90 mins intravenous infusion before oxaliplatin on day 1. Standard antiemetic prophylaxis with a 5HT3-receptor antagonist and dexamethasone were administered to all patients. The inclusion criteria in the present study were patients who completed six cycles of FOLFOX/BEV or four cycles of XELOX/BEV as first-line chemotherapy with a grade 0 or 1 performance status defined by the Eastern Cooperative Oncology Group. The exclusion criteria were patients who did not complete six or four cycles of chemotherapy, or who had received previous chemotherapy. Patients with liver metastases with multiple lesions (four or more) or with lesions greater than five centimeters in the maximum dimension were excluded since these liver metastases can cause liver dysfunction themselves. The splenic volume (SV) was calculated by CT scan volumetry using the sum of the areas of the axial portal venous phase images created by consecutive sequential three millimeter-thick slices. The SV index (SVI) was measured before chemotherapy and after the sixth cycle of chemotherapy as previously described (15). The post-chemotherapeutic CT was performed within four weeks after the sixth cycle of chemotherapy, and patients were excluded if the post-chemotherapeutic CT was performed more than four weeks after the sixth cycle. All patients were evaluated every two or three weeks for adverse events, which were evaluated according to the Common Terminology Criteria for Adverse Events version 4.0 (CTCAE v4.0). The aspartate aminotransferase level, platelet count, and APR were retrieved for the analysis as laboratory markers. The protocol for the present retrospective study was approved by the local ethics committee at our institution and written informed consent had already been obtained from all of the patients. Continuous data were expressed as the means ± standard deviation. Differences between the groups were evaluated by the Mann-Whitney U test, and a P value <0.05 was considered to indicate a statistically significant difference. The data were analyzed using the SPSS software package, version 19.0J.
Results
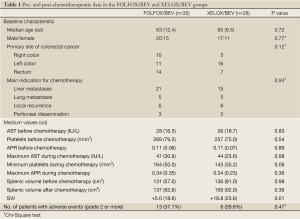
A total of 35 patients receiving FOLFOX/BEV and 28 receiving XELOX/BEV fulfilled the criteria and were evaluated in the present study. No significant differences between the two groups were seen in the data before chemotherapy, including the patient age, gender, primary site of colorectal cancer, the aspartate aminotransferase level, platelet count, APR, SV or indication for chemotherapy (Table 1). However, the SVI in the XELOX/BEV group was significantly higher than that in the FOLFOX/BEV group (P=0.01). The incidence of grade two or higher adverse events did not significantly differ between the two groups, but the platelet count was lower in the XELOX/BEV group than in the FOLFOX BEV group (P=0.08).
Full table
The data analyses were performed according to the previous reports, in which the cut-off values of the SVI and APR were defined at 30% and 0.15, respectively (10,15). The cut-off value of the APR before chemotherapy was set at 0.17 in the previous study, but was set at 0.15 in the present study, because in our 63 patients, the existence of an APR before chemotherapy of 0.15 or higher did not differ between the SVI <30% and SVI ≥30% groups (Table 2).

Full table
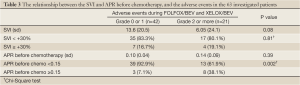
We performed a further analysis of the APR and the SVI with regard to the development of adverse events during chemotherapy. The SVI did not correlate with the incidence of adverse events during chemotherapy. Although the APR before chemotherapy did not significantly differ between the patients with grade 0 or 1 events and those who experienced grade 2 or higher events, the presence of an APR before chemotherapy of 0.15 or higher was significantly more common in the patients who developed grade 2 or higher adverse events (10% vs. 37.5%, P=0.002) (Table 3). The multivariate analysis using a logistic regression method showed that the detection of an APR before chemotherapy of 0.15 or higher was significantly associated with the incidence of adverse events (Table 4).
Full table

Full table
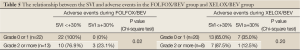
We then analyzed the data regarding the SVI, APR, and adverse events in the FOLFOX/BEV and XELOX/BEV groups. In the FOLFOX/BEV group, the incidence of grade 2 or higher adverse events was significantly higher in the SVI ≥+30% group than in the SVI <+30% group (P=0.02). The incidence of adverse events was also significantly higher in the groups with an APR before chemotherapy ≥0.15 than in the groups with an APR before chemotherapy of <0.15 for both the FOLFOX/BEV group (27.6% vs. 83.3%, P=0.01) and XELOX/BEV group (20.8% vs. 75.0%, P=0.03) (Tables 5,6).
Full table

Full table
Discussion
This study was performed to investigate whether the APR before oxaliplatin-based chemotherapy can predict the development of splenomegaly and adverse events during oxaliplatin-based chemotherapy in patients with stage IV or recurrent colorectal cancer, because our previous study showed that a higher APR before chemotherapy was a predictor of splenomegaly and adverse events (15). In the present study, including the FOLFOX/BEV and XELOX/BEV regimens, we found that an APR before chemotherapy of ≥0.15 can significantly predict the development of adverse events during chemotherapy. Therefore, an APR of ≥0.15 before chemotherapy can be a valuable indicator of whether or not oxaliplatin-based preoperative chemotherapy including BEV should be administered to patients with initially resectable disease when the indication is considered based on the risk of adverse events.
The management of chemotherapy-associated hepatotoxicity is now considered an important issue (7,17) because chemotherapy-associated hepatotoxicity can be an important risk factor for postoperative complications, especially hepatic insufficiency, after a major hepatectomy (9,13). In addition, sinusoidal obstruction syndrome due to oxaliplatin-based chemotherapy may not only compromise the perioperative outcome, but can lead to early recurrence and decreased survival in the long term (18). However, it is still unclear in which cases chemotherapy-associated hepatotoxicity will occur. We previously reported that an indocyanine green retention test, AST before hepatectomy, and completion of six or more cycles of FOLFOX could predict the presence of histopathologic sinusoidal obstruction syndrome (9). Recently, the possible development of splenomegaly and thrombocytopenia induced by portal hypertension due to chemotherapy was reported, and the authors showed that adjuvant FOLFOX significantly increased the SVI in patients with stage II or III colorectal cancer. They suggested that splenomegaly and thrombocytopenia were useful indicators of the presence of chemotherapy-associated liver injury (10,11). Our previous study also highlighted the risk of oxaliplatin–induced splenomegaly in patients receiving FOLFOX (15).
Bevacizumab is now an important component of the treatment for metastatic colorectal cancer, because it improves the chemotherapeutic response and progression-free survival of patients (2,19,20). Overman et al. recently reported that the addition of bevacizumab to oxaliplatin-based chemotherapy reduces the incidence of splenomegaly (11). We previously reported that the SVI was +19.1% in patients treated with FOLFOX (15). Interestingly, in the FOLFOX/BEV group in the present study, the SVI was +5.0% in the FOLFOX/BEV group, confirming the finding reported by Overman et al. They also reported that there was a decreased rate of thrombocytopenia in patients treated with the BEV regimens, and a tendency for this phenomenon was also noticed in our study (149/mm3 in the FOLFOX (15) and 164/mm3 in the FOLFOX/BEV groups, P=0.14, not shown in the Results section). However, no previous study had focused on the SVI in patients receiving the XELOX/BEV regimen.
The present study cannot explain why the SVI was significantly higher in the XELOX/BEV group than in the FOLFOX/BEV. Cassidy et al. found that the incidence of thrombocytopenia was higher in the XELOX group than in the FOLFOX group in the Phase III NO16966 study (7% vs. 3.4% grade 3/4 adverse events) (3). Their results were similar to the present results, in which the platelet counts were lower in the XELOX/BEV group than in the FOLFOX/BEV group. These results seem to be associated with the higher SVI in the XELOX/BEV group than in the FOLFOX/BEV group, because splenomegaly is closely associated with thrombocytopenia (10,11).
Chemotherapy is currently the only treatment available for patients with initially “non-resectable” colorectal liver metastases that can be used to make the disease resectable, because surgical resection following conversion chemotherapy can offer the best chance of cure for these patients (21). Indeed, recent prospective studies have shown the efficacy of conversion chemotherapy using FOLFOX/BEV and XELOX/BEV in patients with initially “non-resectable” colorectal liver metastases (6,22). However, in patients with initially “resectable” colorectal liver metastases, the superiority of preoperative chemotherapy to immediate resection has yet to be fully confirmed. The theoretical advantages of preoperative chemotherapy in patients who are initially resectable include the treatment of undetected distant microscopic metastases, which would reduce the risk of disease recurrence after resection (23). Neoadjuvant chemotherapy may also be useful to determine the chemo-responsiveness of the tumor to help select the optimal adjuvant therapy, as well as identify patients with particularly aggressive disease in whom surgery would be inappropriate (5). On the other hand, a significantly greater morbidity was reported for the EORTC 40983 trial (4), which compared preoperative chemotherapy with immediate surgery in patients with resectable liver metastases. The patients in that study had a postoperative complication rate of 24% in the neoadjuvant group and 13% in the surgery-alone group. In addition, serious adverse events during chemotherapy cannot be disregarded, as shown by several trials in which FOLFOX, XELOX, and bevacizumab were used (6,19,22). Therefore, the indications for preoperative chemotherapy in patients with resectable colorectal liver metastases should be carefully considered from the aspect of oncological advantages, as well as the risk of adverse events. Our previous study showed that an APR before chemotherapy ≥0.17 can predict FOLFOX-induced splenomegaly in patients receiving six cycles of FOLFOX (15).In the present study focusing on BEV-including regimens, an APR before chemotherapy of ≥0.15 was not a predictor of splenomegaly, but was a significant predictor of the development of adverse events during chemotherapy. Therefore, an APR before chemo ≥0.15 can be an important indicator of whether or not oxaliplatin-based preoperative chemotherapy including BEV should be administered for initially resectable disease.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- de Gramont A, Figer A, Seymour M, et al. Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol 2000;18:2938-47.
- Giantonio BJ, Catalano PJ, Meropol NJ, et al. Bevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) for previously treated metastatic colorectal cancer: results from the Eastern Cooperative Oncology Group Study E3200. J Clin Oncol 2007;25:1539-44.
- Cassidy J, Clarke S, Díaz-Rubio E, et al. Randomized phase III study of capecitabine plus oxaliplatin compared with fluorouracil/folinic acid plus oxaliplatin as first-line therapy for metastatic colorectal cancer. J Clin Oncol 2008;26:2006-12.
- Nordlinger B, Sorbye H, Glimelius B, et al. Perioperative chemotherapy with FOLFOX4 and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC Intergroup trial 40983): a randomised controlled trial. Lancet 2008;371:1007-16.
- Blazer DG 3rd, Kishi Y, Maru DM, et al. Pathologic response to preoperative chemotherapy: a new outcome end point after resection of hepatic colorectal metastases. J Clin Oncol 2008;26:5344-51.
- Wong R, Cunningham D, Barbachano Y, et al. A multicentre study of capecitabine, oxaliplatin plus bevacizumab as perioperative treatment of patients with poor-risk colorectal liver-only metastases not selected for upfront resection. Ann Oncol 2011;22:2042-8.
- Rubbia-Brandt L, Audard V, Sartoretti P, et al. Severe hepatic sinusoidal obstruction associated with oxaliplatin-based chemotherapy in patients with metastatic colorectal cancer. Ann Oncol 2004;15:460-6.
- Aloia T, Sebagh M, Plasse M, et al. Liver histology and surgical outcomes after preoperative chemotherapy with fluorouracil plus oxaliplatin in colorectal cancer liver metastases. J Clin Oncol 2006;24:4983-90.
- Nakano H, Oussoultzoglou E, Rosso E, et al. Sinusoidal injury increases morbidity after major hepatectomy in patients with colorectal liver metastases receiving preoperative chemotherapy. Ann Surg 2008;247:118-24.
- Angitapalli R, Litwin AM, Kumar PR, et al. Adjuvant FOLFOX chemotherapy and splenomegaly in patients with stages II-III colorectal cancer. Oncology 2009;76:363-8.
- Overman MJ, Maru DM, Charnsangavej C, et al. Oxaliplatin-mediated increase in spleen size as a biomarker for the development of hepatic sinusoidal injury. J Clin Oncol 2010;28:2549-55.
- Parise ER, Oliveira AC, Figueiredo-Mendes C, et al. Noninvasive serum markers in the diagnosis of structural liver damage in chronic hepatitis C virus infection. Liver Int 2006;26:1095-9.
- Soubrane O, Brouquet A, Zalinski S, et al. Predicting high grade lesions of sinusoidal obstruction syndrome related to oxaliplatin-based chemotherapy for colorectal liver metastases: correlation with post-hepatectomy outcome. Ann Surg 2010;251:454-60.
- Ruzzo A, Graziano F, Loupakis F, et al. Pharmacogenetic profiling in patients with advanced colorectal cancer treated with first-line FOLFIRI chemotherapy. Pharmacogenomics J 2008;8:278-88.
- Miura K, Nakano H, Sakurai J, et al. Splenomegaly in FOLFOX-naïve stage IV or recurrent colorectal cancer patients due to chemotherapy-associated hepatotoxicity can be predicted by the aspartate aminotransferase to platelet ratio before chemotherapy. Int J Clin Oncol 2011;16:257-63.
- Overman MJ, George B, Kalil K, et al. Use of bevacizumab to reduce the rate of oxaliplatin-induced thrombocytopenia and splenomegaly. J Clin Oncol 2012;30:abstr 564.
- Chun YS, Laurent A, Maru D, et al. Management of chemotherapy-associated hepatotoxicity in colorectal liver metastases. Lancet Oncol 2009;10:278-86.
- Tamandl D, Klinger M, Eipeldauer S, et al. Sinusoidal obstruction syndrome impairs long-term outcome of colorectal liver metastases treated with resection after neoadjuvant chemotherapy. Ann Surg Oncol 2011;18:421-30.
- Gruenberger B, Tamandl D, Schueller J, et al. Bevacizumab, capecitabine, and oxaliplatin as neoadjuvant therapy for patients with potentially curable metastatic colorectal cancer. J Clin Oncol 2008;26:1830-5.
- Saltz LB, Clarke S, Díaz-Rubio E, et al. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study. J Clin Oncol 2008;26:2013-9.
- Adam R, de Haas RJ, Wicherts DA, et al. Concomitant extrahepatic disease in patients with colorectal liver metastases: when is there a place for surgery? Ann Surg 2011;253:349-59.
- Bertolini F, Malavasi N, Scarabelli L, et al. FOLFOX6 and bevacizumab in non-optimally resectable liver metastases from colorectal cancer. Br J Cancer 2011;104:1079-84.
- Ellis LM, Curley SA, Grothey A. Surgical resection after downsizing of colorectal liver metastasis in the era of bevacizumab. J Clin Oncol 2005;23:4853-5.


