Can only chemoradiotherapy and chemotherapy treatment be applied to patients with rectal cancer who could not be operated?
Introduction
Colorectal cancer is a common and fatal disease. Approximately 148,810 new cases are detected each year. In the USA, and 108,070 of those have colon cancer and the others have rectal cancer (1). In terms of frequency it is the third disease in both females and males and it is the third leading cause of death. Colorectal cancers constitute 10% of all cancer cases and it is responsible for 10% of all cancer related deaths (2). Main treatment option for colorectal cancer is the surgery. Adjuvant chemotherapy (CT) is recommended for patients with stage II disease keeping certain risk factors and for all stage III patients. Some of the patients with stage IV disease are treated following patient-based evaluations (2-4).
Surgery is the main treatment option in rectal cancer. Afterwards, adjuvant treatment methods were investigated to increase the efficacy, and the initial researches were focused on adjuvant radiotherapy (RT), which demonstrated to decrease the recurrence rates (5). The following studies has shown that adjuvant chemoradiotherapy (CRT) is more efficient compared to adjuvant RT and this approach decreased both local recurrences (6) and cancer related deaths (7,8). The ongoing studies revealed that neoadjuvant RT had better control on local recurrences compared with adjuvant RT (9), and the neoadjuvant CRT is superior to neoadjuvant RT in prevention of local recurrences and upward trend in survival, therefore neoadjuvant CRT was considered as the most appropriate approach (10-13). CT, another treatment option in rectal cancer, was also showed to be effective and it significantly increased the survival (5,14-16). Thus, the multimodal approach in which the surgery, neoadjuvant CRT and adjuvant CT are administered in combination generated the most optimal approach in the treatment of locally advanced stage rectal cancer (17,18). Particularly, the neoadjuvant administration of CRT provided benefits in terms of sphincter prevention and quality of life (11-13,18-20). Also, patients with locally advanced stage rectal cancer are treated by this approach in our department.
In the literature, it has been agreed that surgery is the main treatment method for rectal cancer. However, surgery cannot be administered in some patients due to various reasons. Treatment with CRT and CT, which are the significant components of multimodal treatment, might be discussed for such patients. The data of the patients who could not undergo surgery due to any reason and who were followed up after receiving only CRT or CT following CRT, have not been completely presented yet. We have planned this study to evaluate the characteristics of the patients who had been diagnosed with locally advanced stage non-metastatic rectal cancer in their initial evaluations and who had not undergone surgery due to any reason but only received CRT or CT following CRT.
Materials and methods
Patients with locally advanced stage non-metastatic rectal cancer, who were treated and followed-up in Dokuz Eylul University, Medical Faculty, Department of Internal Diseases, Division of Medical Oncology between January 1999 and August 2009 were evaluated. Patients’ files were retrospectively reviewed and data were recorded. Characteristics of patients, who were not operated due to any reason and treated with CRT alone or CT following CRT, were assessed.
Patients with stage II and III rectal cancer, according to American Joint Committee on Cancer’s (AJCC) Cancer Staging 6th edition 2002 TNM staging system (21) were included in the study. Accordingly, T3-4N0/N+ was considered locally advanced and, T3-4N0 was considered of stage II, as N+ was stage III.
Preoperative evaluations were performed by thoracic, lower, and upper abdominal computerized tomography (CT), lower abdominal (pelvic) magnetic resonance imaging (MRI), and endorectal ultrasound (US) studies in all patients. Absence of distant metastasis was confirmed by thoracic, upper, and lower abdominal CT and/or positron emission tomography-computerized tomography (PET-CT).
The patients receiving CRT were administered RT in 1.8 Gy/fraction/day dosage for 25 fractions, a total of 45 Gy and in addition they were given 5-fluorourasil (5-FU) 225 mg/m2/day as continuous infusion. The dosage of oxaliplatin was 50 mg/m2/day in cases who received oxaliptalin in addition to RT and 5-FU in CRT protocol. Capecitabine was administered with a dosage of 1,000 mg/m2 every day in cases who received capecitabine instead of 5-FU in CRT protocol. Following CRT, capecitabine was administered as monotherapy with a dosage of 2,500 mg/m2/d for 14 days followed by a 7 day rest. Following CRT, CT was administered in a modified FOLFOX6 regimen was given once in 14 days, including folinic acid 400 mg/m2 + 5-FU 400 mg/m2 bolus + 5-FU 2,400 mg/m2 46 hours of infusion + oxaliplatin 85 mg/m2.
Time from diagnosis to progression was defined as progression free survival (PFS) and time from diagnosis to death was defined as overall survival (OS).
The statistical analyses of the data were performed by Statistical Package for Social Sciences for Windows (SPSS) Version 15.0 software; and Kaplan-Meier Method was used for PFS and OS analyses.
Results
The retrospective analyses of 263 patients with rectal cancer were performed. 86 patients (32.6%) with stage II and 177 patients (67.4%) with stage III rectal cancer had a median age of 59 [18-85] years. The patient characteristics are presented in Table 1.
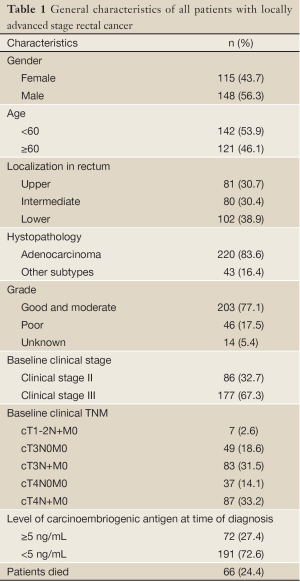
Full table
Among those, 14 patients (5.3%) were determined who could not undergo surgery due to any reason, but received CRT or CT following CRT. 4 of them were women (28.6%) and 10 were men (71.4%) and the median age was 72 [42-87] years. All of these 14 patients had CRT, and additional CT was received by 2 (14.3%) patients. In the CRT protocol, 12 of 14 patients received continuous infusion 5-FU, one patients received oxaliplatin in addition to 5-FU and RT and, one patients received capecitabine instead of 5-FU. In CT protocol, one patient received 12 courses of modified FOLFOX6 and one patient received 7 courses of capecitabine. This 14 patients characteristics are presented in Table 2.
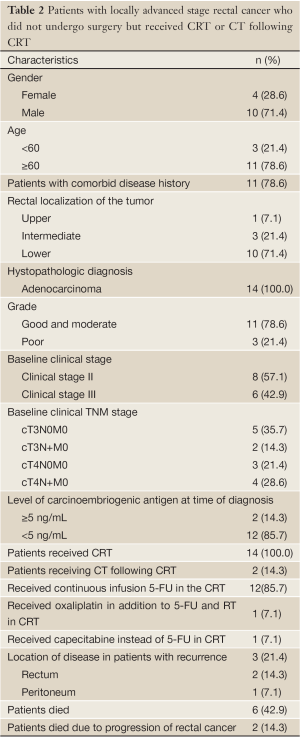
Full table
Most of the patients were elders and 11 (78.6%) were 60 and older and 7 (50.0%) of these eleven patient were 70 or older. The baseline examinations revelaed that 8 patients (57.1%) had stage II and 6 patients (42.9%) had stage III disease. 3 of these patients were inappropriate for surgery due to advanced age and health status, and the other 11 patients did not want to undergo surgery on their own account. The main reason for their refusal of the surgery was their advanced age.
3 patients had no comorbid diseases, but 8 patients (57.1%) had hypertension, 5 (35.7%) had coronary artery disease, 4 (28.6%) had diabetes mellitus, 3 (21.4%) had chronic obstructive lung disease, 1 (7.1%) had chronic renal disease, 1 (7.1%) had history of cerebrovascular disease and 1 (7.1%) had history of peripheral vascular disease.
The evaluation of rectal tumor localizations of the patients demonstrated that localization in the lower rectum was more frequent. The histopathologic diagnosis was adenocarcinoma in all of the patients. Following the histopathologic diagnosis, the carcinoembriogenic antigen (CEA) levels were normal in 12 patients (85.7%) and greater than 5 ng/mL in 2 patients (14.3%).
Recurrence was determined in 3 (21.4%) patients. All three patients was stage II. 2 of them had local recurrence and 1 had peritoneal carcinomatous recurrence. There was no recurrence detected in any patients receiving CT following CRT. Patients with local recurrence accepted surgery after diagnosis of the recurrence and they underwent operation. 6 patients (42.9%) died. Five patients was stage II and one stage III. 2 deaths (14.3%) were determined due to the progression of rectal cancer. These patients was stage II. The other deaths were due to non-cancer reasons.
The median PFS and OS were 25 [8-68] and 35 [12-68] months, respectively. Moreover, 1, 3 and 5-year OS rates were 92.9%, 69.8% and 52.4%, respectively.
Discussion
The multimodal approach consisting of neoadjuvant CRT, surgery and adjuvant CT is widely accepted as an optimal treatment in locally advanced stage rectal cancer. Surgery is the main treatment step in this approach. However, neoadjuvant CRT or CT following CRT is an appropriate treatment option for patients who are not eligible for surgery due to any reason. Therefore, we presented these 14 patients to evaluate the disease progression in patients that surgery cannot be performed.
We have determined that the prognosis of patients with non-metastatic locally advanced stage rectal cancer who could not be operated but received only CRT or CT following CRT were not worse than those that underwent surgical treatment. 3 (21.4%) of these patients had advanced age and poor performance status for surgery, and 11 of them refused undergoing an operation. The main reason for the patients’ rejection of surgery was their advanced ages. Only 3 (21.4%) of 14 patients experienced recurrence and only 2 (14.3%) patients died due to disease progression. 2 of 3 patients with recurrence had operable rectal cancer recurrence and one had peritoneal carcinomatous relapse. We have determined that PFS was over 2 years and OS was up to 3 years.
The outcomes of treatment in locally advanced stage rectal cancer may vary according to the methods in the literature. In spite of advances in surgical techniques and routinely applied total mesorectal excision, the survival rates in patients with only surgical treatment is less than 50%, however, it can rise up to 80% in patients receiving neoadjuvant CRT and adjuvant CT in addition to surgical treatment. Locally advanced stage rectal cancer, despite the proven efficacy of the addition of CRT and CT to surgical treatment in patients receiving all three treatments, this rate is still high recurrence rates, significant levels with 25-50% (5,11-20). The patients included in our study had not undergone surgical treatment, however, 1, 3 and 5-year OS rates were 92.9%, 69.8% and 52.4% and the local recurrence rates were 14.2%, and compared to the which undergone surgical treatment patients in the literature the outcomes were reasonable, suggesting that administering CRT followed by CT is an appropriate treatment option for patients who could not be operated due to any other reason.
Eleven (78.6%) of 14 patients in our study had comorbid diseases and 4 of 6 patients died due non-cancer reasons. Although the surgical methods used in rectal cancer show significant variations among centers in the literature, the morbidity rate is approximately 30% and the mortality rate is 2%, and these methods result hospitalization up to 3 to 45 days (22-24). When considering all of these outcomes, it seems that CRT with a less morbidity rate is an alternative treatment option instead of surgical treatment in patients with advanced age and comorbid diseases.
Although there are a limited number of studies demonstrating that adjuvant CT is another important treatment in rectal cancer, it was shown that patients in the CT arm had better survival compared with the other arms (5). The following studies revealed that patients receiving CT had less recurrences and death rates compared with the non-receivers (8,14). On the other hand, it was shown that orally administered adjuvant CT instead of parenteral CT also increase survival in patients with locally advanced stage rectal cancer (15). In our study some of the patients had received capecitabine.
Since our study is a retrospective study, it has the specific deficits of retrospective studies. However, we presented this study to report that eligible patients who cannot undergo surgery can be followed up after receiving CRT and CRT followed by CT in the treatment of locally advanced stage rectal cancer, thus it may be an optional treatment in such patients.
Surgery is the main treatment modality in rectal cancer. Therefore, in this study, the aim is not to present data on the efficacy of surgical treatment. We investigated the effectiveness of treatment methods other than surgical treatment. Consequently we consider that only CRT or CT following CRT may be administered in patients with locally advanced rectal cancer who cannot undergo surgical treatment due to advanced age, poor performance status, significant comorbid diseases, surgery refusals or not operable due to any other reason.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin 2008;58:71-96.
- Steven KL, Leonardo BS, Joel ET. Colon Cancer. In: DeVita VT, Lawrence TS, Rosenberg SA. eds. DeVita, Hellman, and Rosenberg’s Cancer Principles & Practice of Oncology. 8th ed. Philadelphia: Lippincott Williams&Wilkins, 2008:1232-85.
- Benjamin RT. Gastrointestinal Cancer: Colorectal And Anal. In: Ramaswamy Govindan, eds. The Washington Manual Of Oncology. 2nd ed. Philadelphia: Lippincott Williams&Wilkins, 2008:190-6.
- O’Neil BH, Goldberg RM. Innovations in chemotherapy for metastatic colorectal cancer: an update of recent clinical trials. Oncologist 2008;13:1074-83.
- Fisher B, Wolmark N, Rockette H, et al. Postoperative adjuvant chemotherapy or radiation therapy for rectal cancer: results from NSABP protocol R-01. J Natl Cancer Inst 1988;80:21-9.
- Prolongation of the disease-free interval in surgically treated rectal carcinoma. Gastrointestinal Tumor Study Group. N Engl J Med 1985;312:1465-72.
- Krook JE, Moertel CG, Gunderson LL, et al. Effective surgical adjuvant therapy for high-risk rectal carcinoma. N Engl J Med 1991;324:709-15.
- Wolmark N, Wieand HS, Hyams DM, et al. Randomized trial of postoperative adjuvant chemotherapy with or without radiotherapy for carcinoma of the rectum: National Surgical Adjuvant Breast and Bowel Project Protocol R-02. J Natl Cancer Inst 2000;92:388-96.
- Gérard A, Buyse M, Nordlinger B, et al. Preoperative radiotherapy as adjuvant treatment in rectal cancer. Final results of a randomized study of the European Organization for Research and Treatment of Cancer (EORTC). Ann Surg 1988;208:606-14.
- Carlomagno C, Pepe S, D’Armiento FP, et al. Predictive factors of complete response to neoadjuvant chemoradiotherapy in patients with rectal cancer. Oncology 2010;78:369-75.
- Chari RS, Tyler DS, Anscher MS, et al. Preoperative radiation and chemotherapy in the treatment of adenocarcinoma of the rectum. Ann Surg 1995;221:778-86; discussion 786-7.
- Sauer R, Becker H, Hohenberger W, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med 2004;351:1731-40.
- Roh MS, Colangelo LH, O’Connell MJ, et al. Preoperative multimodality therapy improves disease-free survival in patients with carcinoma of the rectum: NSABP R-03. J Clin Oncol 2009;27:5124-30.
- Quasar Collaborative Group, Gray R, Barnwell J, et al. Adjuvant chemotherapy versus observation in patients with colorectal cancer: a randomised study. Lancet 2007;370:2020-9.
- Akasu T, Moriya Y, Ohashi Y, et al. Adjuvant chemotherapy with uracil-tegafur for pathological stage III rectal cancer after mesorectal excision with selective lateral pelvic lymphadenectomy: a multicenter randomized controlled trial. Jpn J Clin Oncol 2006;36:237-44.
- O’Connell MJ, Martenson JA, Wieand HS, et al. Improving adjuvant therapy for rectal cancer by combining protracted-infusion fluorouracil with radiation therapy after curative surgery. N Engl J Med 1994;331:502-7.
- Steven KL, Joel ET, Leonardo BS. Rectal Cancer. In: DeVita VT, Lawrence TS, Rosenberg SA. eds. DeVita, Hellman, and Rosenberg’s Cancer Principles & Practice of Oncology. 8th ed. Philadelphia: Lippincott Williams&Wilkins, 2008:1285-301.
- Das P, Skibber JM, Rodriguez-Bigas MA, et al. Clinical and pathologic predictors of locoregional recurrence, distant metastasis, and overall survival in patients treated with chemoradiation and mesorectal excision for rectal cancer. Am J Clin Oncol 2006;29:219-24.
- Citrin D, Camphausen K, Wood BJ, et al. A pilot feasibility study of TNFerade™ biologic with capecitabine and radiation therapy followed by surgical resection for the treatment of rectal cancer. Oncology 2010;79:382-8.
- Mendenhall WM, Zlotecki RA, Snead FE, et al. Radiotherapy in the treatment of resectable rectal adenocarcinoma. Am J Clin Oncol 2009;32:629-38.
- American Joint Committee on Cancer. Colon and Rectum. Philadelphia: Lippincott-Raven Publishers, 2002.
- Celiku E, Draçini X, Dibra A, et al. Rectal cancer surgery. A ten years experience. G Chir 2010;31:507-10.
- Zaheer S, Pemberton JH, Farouk R, et al. Surgical treatment of adenocarcinoma of the rectum. Ann Surg 1998;227:800-11.
- Osler M, Iversen LH, Borglykke A, et al. Hospital variation in 30-day mortality after colorectal cancer surgery in denmark: the contribution of hospital volume and patient characteristics. Ann Surg 2011;253:733-8.


